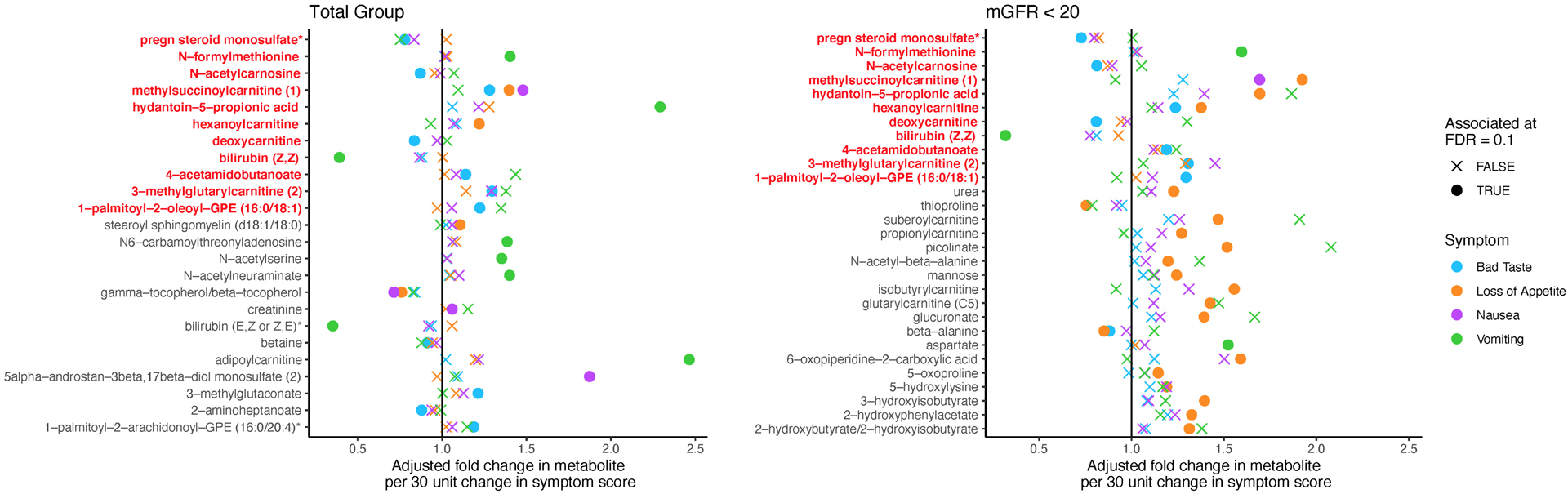
Practice Essentials
Uremic encephalopathy is an organic brain disorder. It develops in patients with acute kidney injury or chronic kidney disease, usually when the estimated glomerular filtration rate (eGFR) falls and remains below 15 mL/min.
Manifestations of this syndrome vary from mild symptoms (eg, lassitude, fatigue) to severe signs (eg, seizures, coma). Severity and progression depend on the rate of decline in kidney function; thus, symptoms are usually worse in patients with acute kidney injury. Prompt identification of uremia as the cause of encephalopathy is essential because symptoms are readily reversible following initiation of dialysis.
Pathophysiology
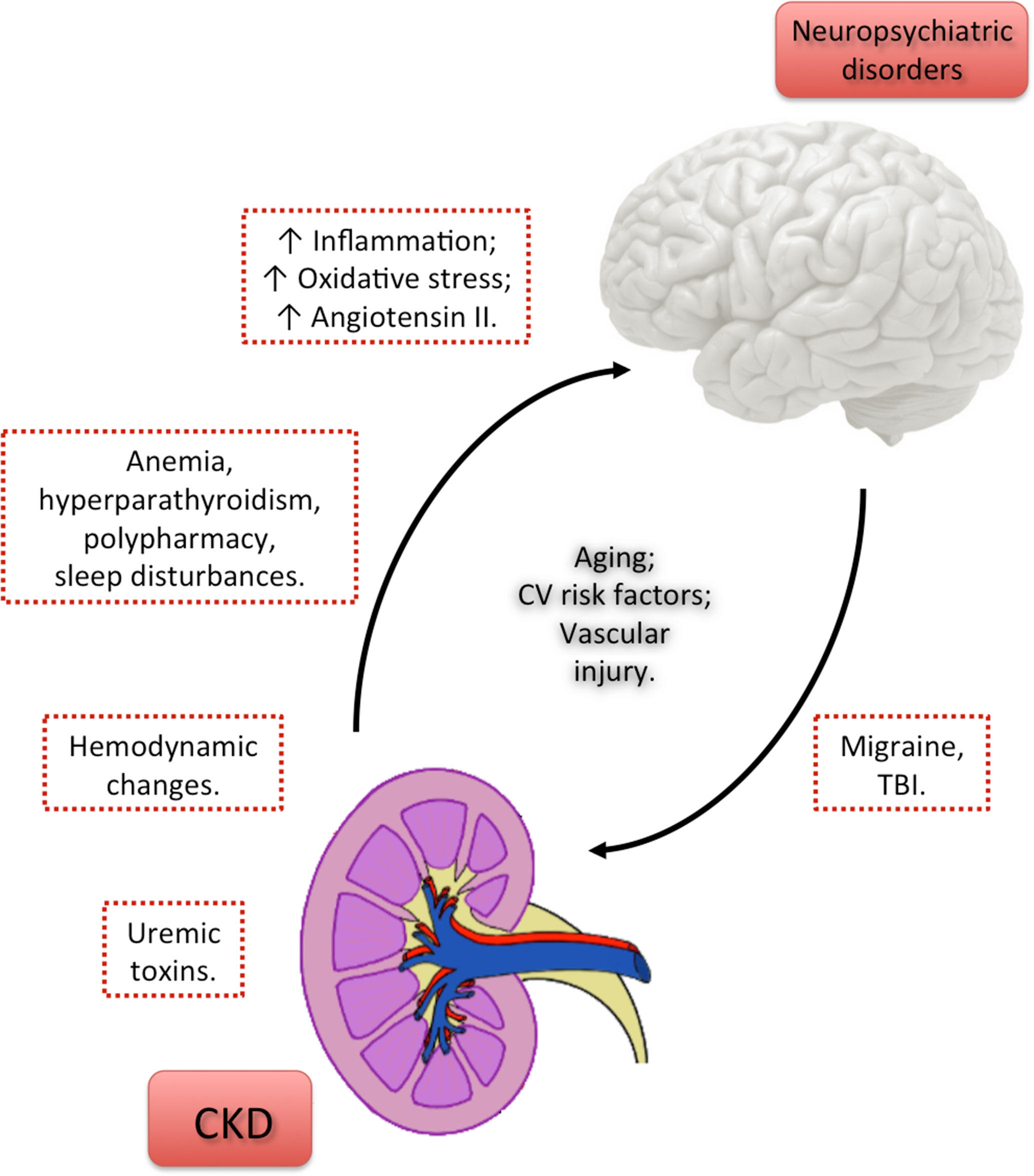
Uremic encephalopathy has a complex pathophysiology, and many toxins that accumulate in kidney failure may be contributive. Uremic encephalopathy may occur in a patient with acute kidney injury or chronic kidney failure of any etiology. Likely causes include the following :
- Retention of uremic solutes
- Alterations in hormonal metabolism
- Changes in electrolyte and acid-base homeostasis
- Increased vascular reactivity, blood-brain barrier transport, and inflammation
One contributing factor to uremic encephalopathy may involve imbalances of neurotransmitter amino acids within the brain. During the early phase of uremic encephalopathy, plasma and cerebrospinal fluid (CSF) determinations indicate that levels of glycine increase and levels of glutamine and gamma-aminobutyric acid (GABA) decrease. As uremia progresses, it has been proposed that the accumulation of guanidino compounds results in activation of excitatory N-methyl-D-aspartate (NMDA) receptors and inhibition of inhibitory GABA receptors, which may cause myoclonus and seizures. In addition, alterations occur in metabolism of dopamine and serotonin in the brain, which may lead to early symptoms (eg, sensorial clouding).
Parathyroid hormone (PTH) likely contributes to uremic encephalopathy. Secondary hyperparathyroidism, which occurs in kidney failure, causes an increase in calcium content in the cerebral cortex. In animal models with uremia, electroencephalographic (EEG) changes were typical of those observed in patients with renal failure. In uremic patients with secondary hyperparathyroidism, EEG changes have been shown to improve after medical suppression of PTH or parathyroidectomy.
The specific mechanism by which PTH causes disturbance in brain function is unclear, but it may involve increases in intracellular concentration of calcium in brain cells. However, since the encephalopathy improves with dialysis, which does not have a marked effect on PTH levels, hyperparathyroidism is not thought to be the main cause.
A study of acute kidney injury in mice found evidence of a blood-brain barrier disruption from such injury, with increased neuronal pyknosis and microgliosis. In addition, proinflammatory chemokines were increased in brain tissue.
Numerous other uremic toxins may contribute to uremic encephalopathy, but there has been a notable lack of research in this area. Although the encephalopathy correlates roughly with blood urea nitrogen (BUN) level, urea is not itself thought to be causative.
Epidemiology
Most patients with an eGFR of less than 10 mL/min develop some degree of encephalopathy; however, they may not be clearly symptomatic. In one pediatric study, encephalopathy occurred in 40% of the children with a BUN level greater than 90 mg/dL. As the BUN level increased, the likelihood of these children developing convulsions increased.
No racial predilection exists. No significant association between sex and incidence exists. Uremic encephalopathy may develop at any age.
Prognosis
Symptoms include somnolence and decreased mentation. Asterixis may be present. These findings are reversible following initiation of dialysis and recovery of kidney function in patients with acute kidney injury. Symptoms are also reversible following the institution of dialysis or kidney transplantation in patients with end-stage renal disease (ESRD).
The severe complications (ie, seizures, coma) can lead to death. Early recognition of encephalopathy in the setting of decreased kidney function is crucial to prevent morbidity or mortality. With prompt dialytic therapy, the mortality rate is low.
History
Early symptoms of uremic encephalopathy include the following:
- Anorexia
- Nausea
- Restlessness
- Drowsiness
- Diminished ability to concentrate
- Slowed cognitive functions
More severe signs and symptoms of uremic encephalopathy include the following:
- Vomiting
- Emotional volatility
- Decreased cognitive function
- Disorientation
- Confusion
- Bizarre behavior
- Stupor, coma
Physical Examination
Physical examination findings may include the following:
- Altered mental status (confusion)
- Cranial nerve signs (nystagmus)
- Papilledema
- Hyperreflexia, clonus, asterixis
- Altered gait
- Stupor
- Coma (occurs only if uremia remains untreated and progresses)
Differential Diagnoses
Hepatic Encephalopathy
Hypercalcemia
Hypermagnesemia
Hypernatremia
Hyperosmolar Coma
Hyperparathyroidism
Hypertensive Encephalopathy
Hypoglycemia
Hyponatremia
Hypophosphatemia
Posterior reversible encephalopathy syndrome
Subdural Hematoma Surgery
Wernicke-Korsakoff Syndrome
Laboratory Studies
Obtain the following laboratory studies:
Kidney function studies – Markedly elevated blood urea nitrogen (BUN) and creatinine levels are seen in uremic encephalopathy
Serum electrolyte and glucose measurements – To rule out hyponatremia, hypernatremia, hyperglycemia, and hyperosmolar syndromes as the cause of encephalopathy
Complete blood cell count – To detect leukocytosis, which may suggest an infectious cause, and to determine whether anemia is present (anemia may contribute to the severity of mental alterations)
Serum calcium, phosphate, and parathyroid hormone (PTH) levels – To assess for hypercalcemia, hypophosphatemia, and severe hyperparathyroidism, which cause metabolic encephalopathy
Serum magnesium level – This may be elevated in a patient with renal insufficiency, particularly if the patient is ingesting magnesium-containing antacids; hypermagnesemia may manifest as encephalopathy
Toxicology screen
Medication levels
Determine drug levels because medications (eg, digoxin, lithium) may accumulate in patients with kidney failure and contribute to encephalopathy. However, some medications that are excreted by the kidney cannot be detected. These may also accumulate in patients with kidney failure, resulting in encephalopathy (eg, penicillin, cimetidine, meperidine, baclofen).
Imaging Studies
Obtain a magnetic resonance imaging (MRI) or computed tomography (CT) scan of the head in uremic patients who present with severe neurologic symptoms, to rule out structural abnormalities (eg, stroke, intracranial mass, subdural hematoma).
Typical MRI findings in patients with uremic encephalopathy include increased signal intensity (lentiform fork sign) in either the cerebral cortex or the basal ganglia. A CT scan may show bilateral hypodensities involving the basal ganglia, midbrain, or thalamus.
In a prospective study of 20 patients diagnosed with uremic encephalopathy, MRI scans did not show basal ganglia findings, and the lentiform fork sign was not observed. However, in the majority of patients the MRI showed white matter involvement and cerebral or cortical atrophy, and in half the patients, arterial blood gas (ABG) analysis revealed metabolic acidosis. The researchers concluded that while the lentiform fork sign and basal ganglia involvement can confirm the diagnosis, uremic encephalopathy cannot be ruled out in its absence; in those cases, clinical manifestations and laboratory findings need to be taken into account.
Encephalography
An electroencephalograpm (EEG) is commonly performed on patients with metabolic encephalopathy. Findings typically include the following:
- Slowing and loss of alpha frequency waves
- Disorganization
- Intermittent bursts of theta and delta waves with slow background activity
Reduction in frequency of EEG waves correlates with the decrease in renal function and the alterations in cerebral function. After the initial period of dialysis, clinical stabilization may occur while the EEG findings do not improve. Eventually, EEG results move toward normal.
Aside from the routine EEG, evoked potentials (EPs) (ie, EEG signals that occur at a reproducible time after the brain receives a sensory stimulus [eg, visual, auditory, somatosensory]) may be helpful in evaluating uremic encephalopathy. Chronic renal failure prolongs latency of the cortical visual-evoked response. Auditory-evoked responses are generally not altered in uremia, but delays in the cortical potential of the somatosensory-evoked response do occur.
Cognitive Function Tests
Several cognitive function tests are used to evaluate uremic encephalopathy, including the following:
- Trail-making test – Measures psychomotor speed; uremia may result in worse performance
- Continuous memory test – Measures short-term recognition
- Choice reaction time test – Measures simple decision making
Alterations in choice reaction time appear to correlate best with renal failure.
Procedures
Lumbar puncture is not routinely performed; however, it may be indicated to find other causes of encephalopathy if a patient’s mental status does not improve after initiation of dialysis. No specific CSF finding indicates uremic encephalopathy.
Approach Considerations
The presence of uremic encephalopathy in a patient with either acute kidney injury or chronic kidney disease is an indication for the initiation of dialytic therapy (ie, hemodialysis, peritoneal dialysis, continuous renal replacement therapy). Yanai et al reported three cases of uremic encephalopathy that developed in anuric patients receiving peritoneal dialysis; all cases resolved with institution of hemodialysis.
After beginning dialysis, patients generally show clinical improvement, although electroencephalographic (EEG) findings may not improve immediately. In patients with end-stage renal disease (ESRD), EEG abnormalities generally improve after several months but may not completely normalize.
Address the following factors when treating uremic encephalopathy, which are also included in the standard care of any patient with ESRD:
- Adequacy of dialysis
- Correction of anemia
- Regulation of calcium and phosphate metabolism
Administer medications (eg, iron, erythropoietin, phosphate binders, vitamin D analogues) for patients with ESRD to optimize their quality of life. Sedatives should be avoided.
Consultations
Consult a neurologist if symptoms do not improve upon initiation of dialysis. Consult a vascular surgeon for placement of vascular access in patients with ESRD. Refer patients with ESRD to a dietitian familiar with renal diseases. Refer patients with chronic kidney disease to a nephrologist for regular monitoring of estimated glomerular filtration rate (eGFR), so that dialysis may be initiated before encephalopathy develops.
Diet
To avoid malnutrition in patients with ESRD, maintain adequate protein intake (1.2 g/kg/d) and initiate dialysis (despite the presence of encephalopathy).
Long-Term Monitoring
Schedule maintenance hemodialysis for patients who have ESRD.
Mental status should be carefully monitored.