Practice Essentials
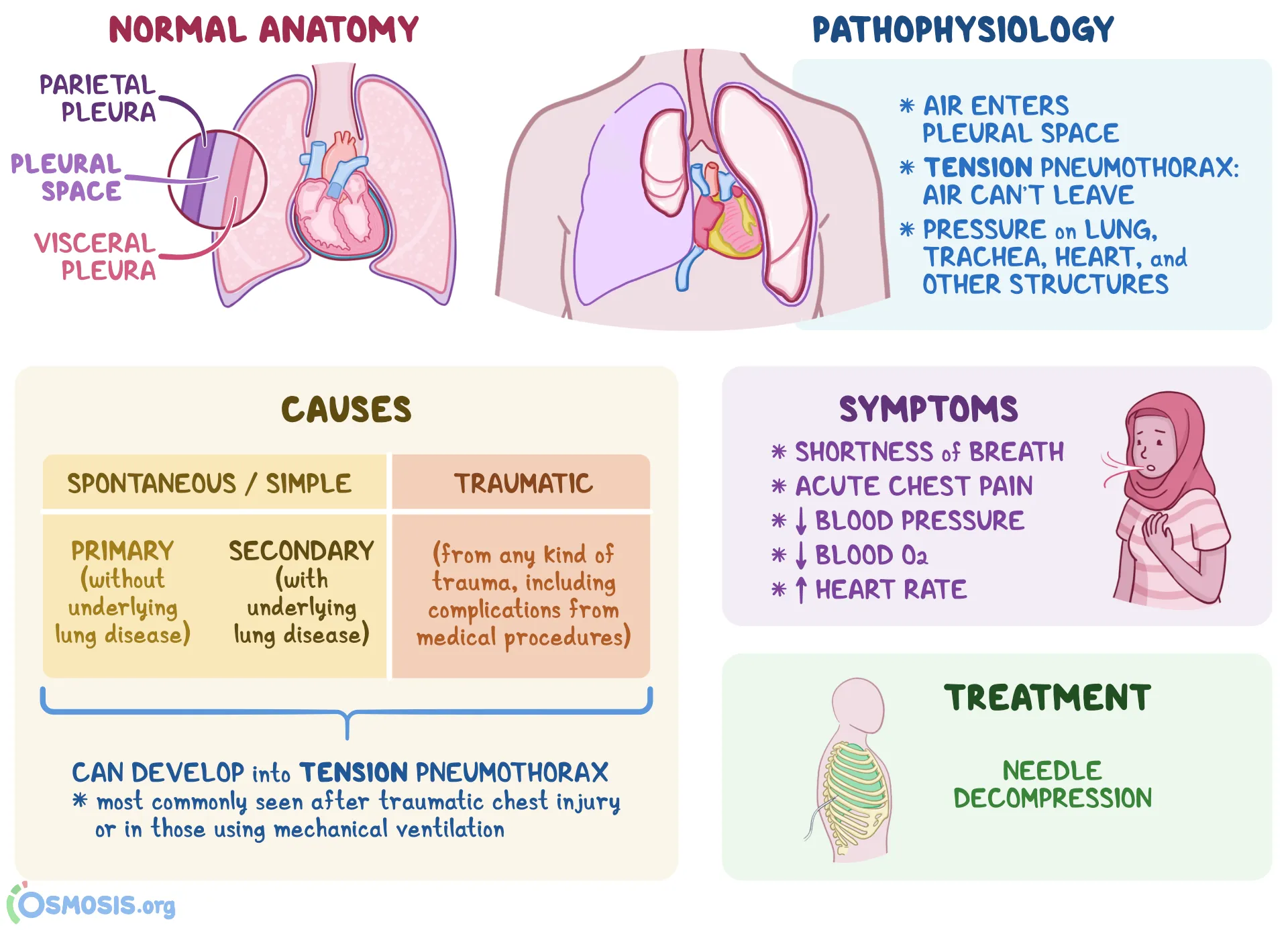
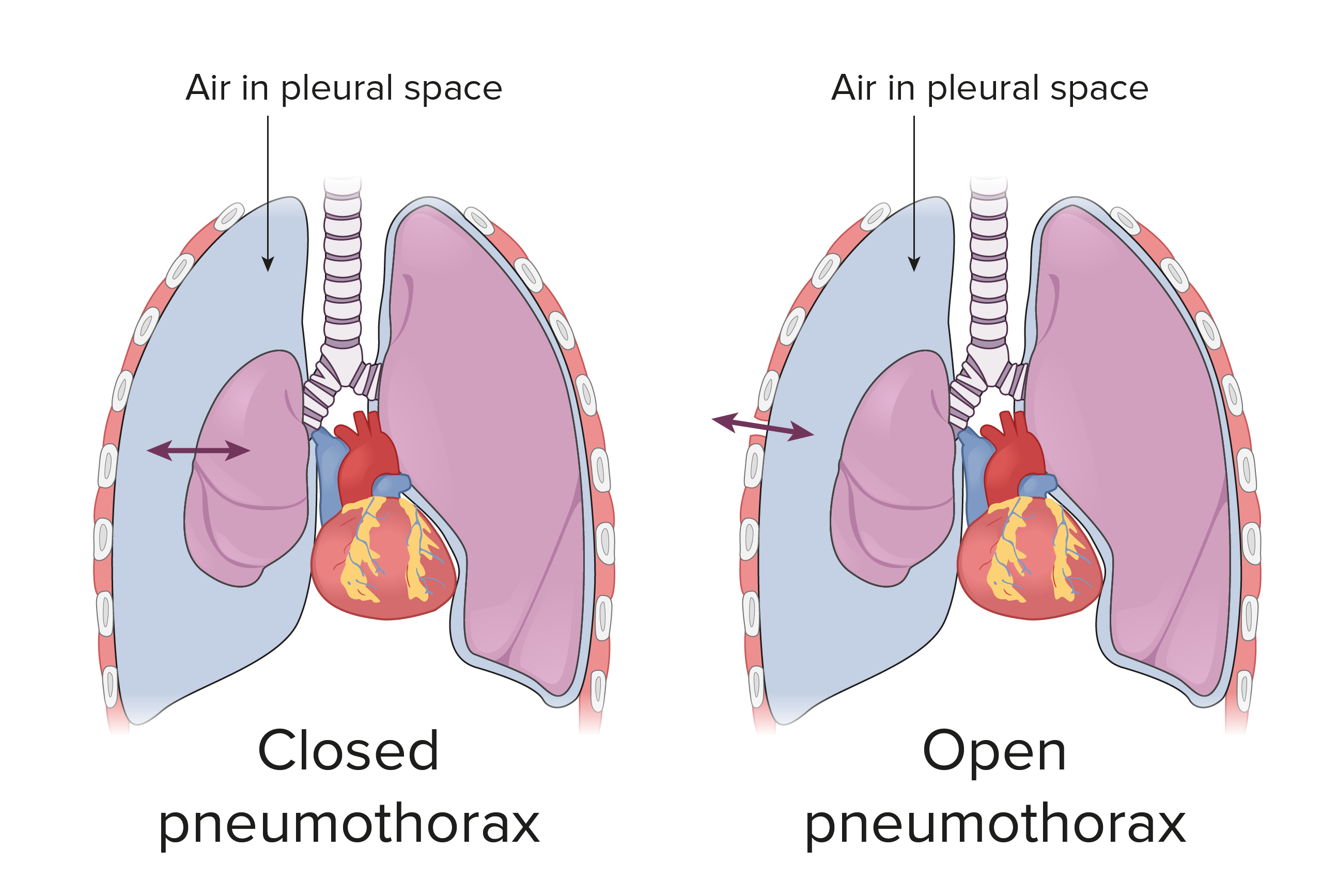
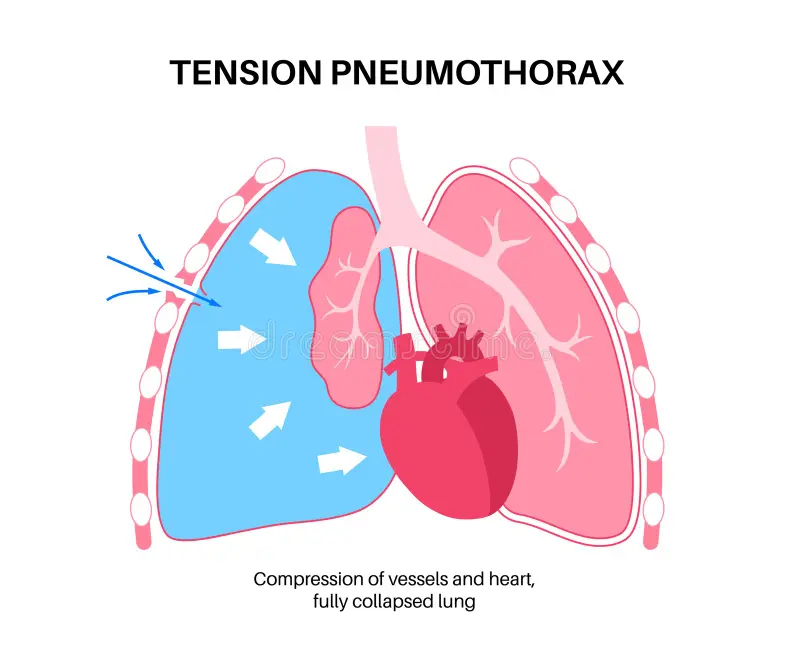
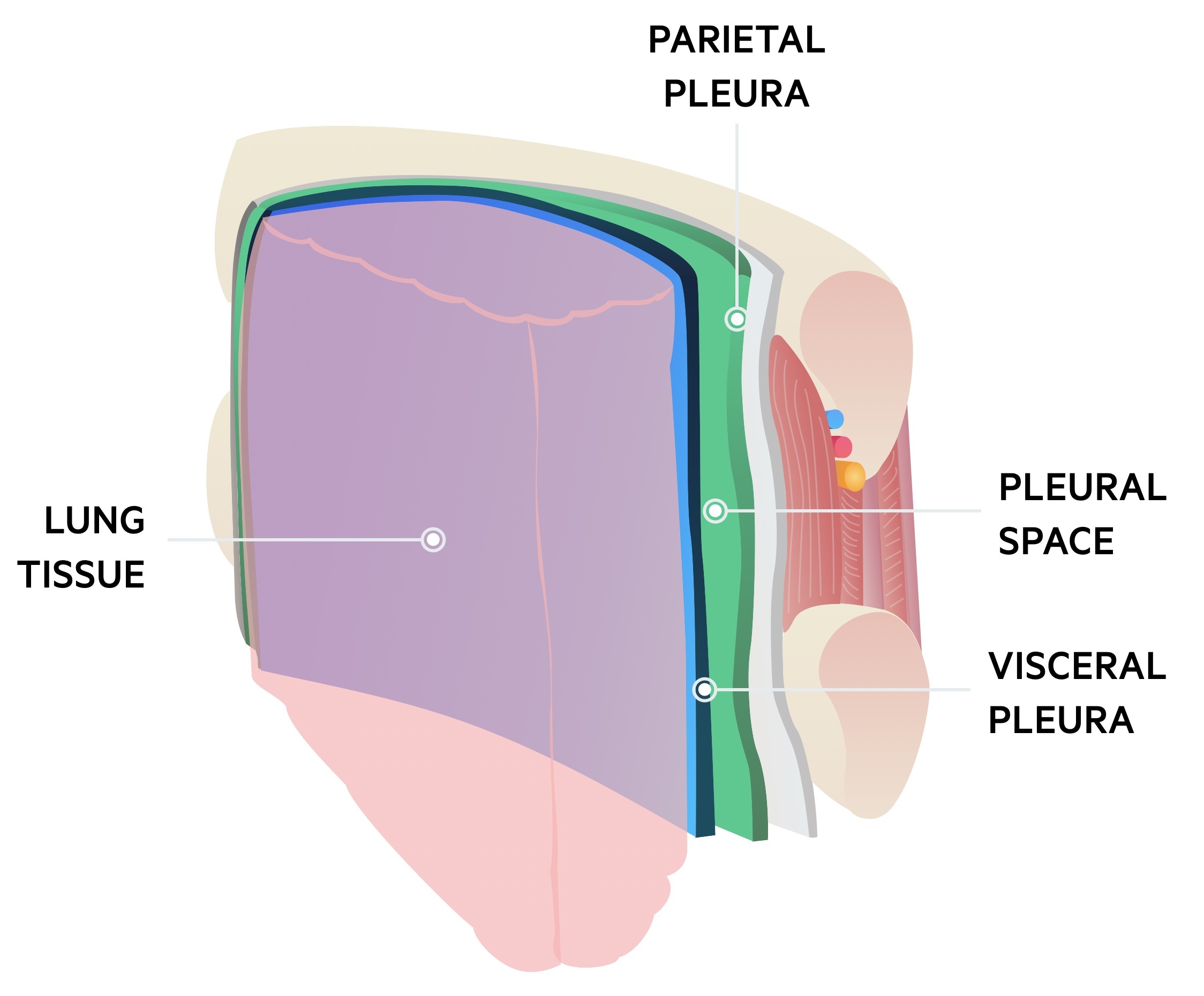
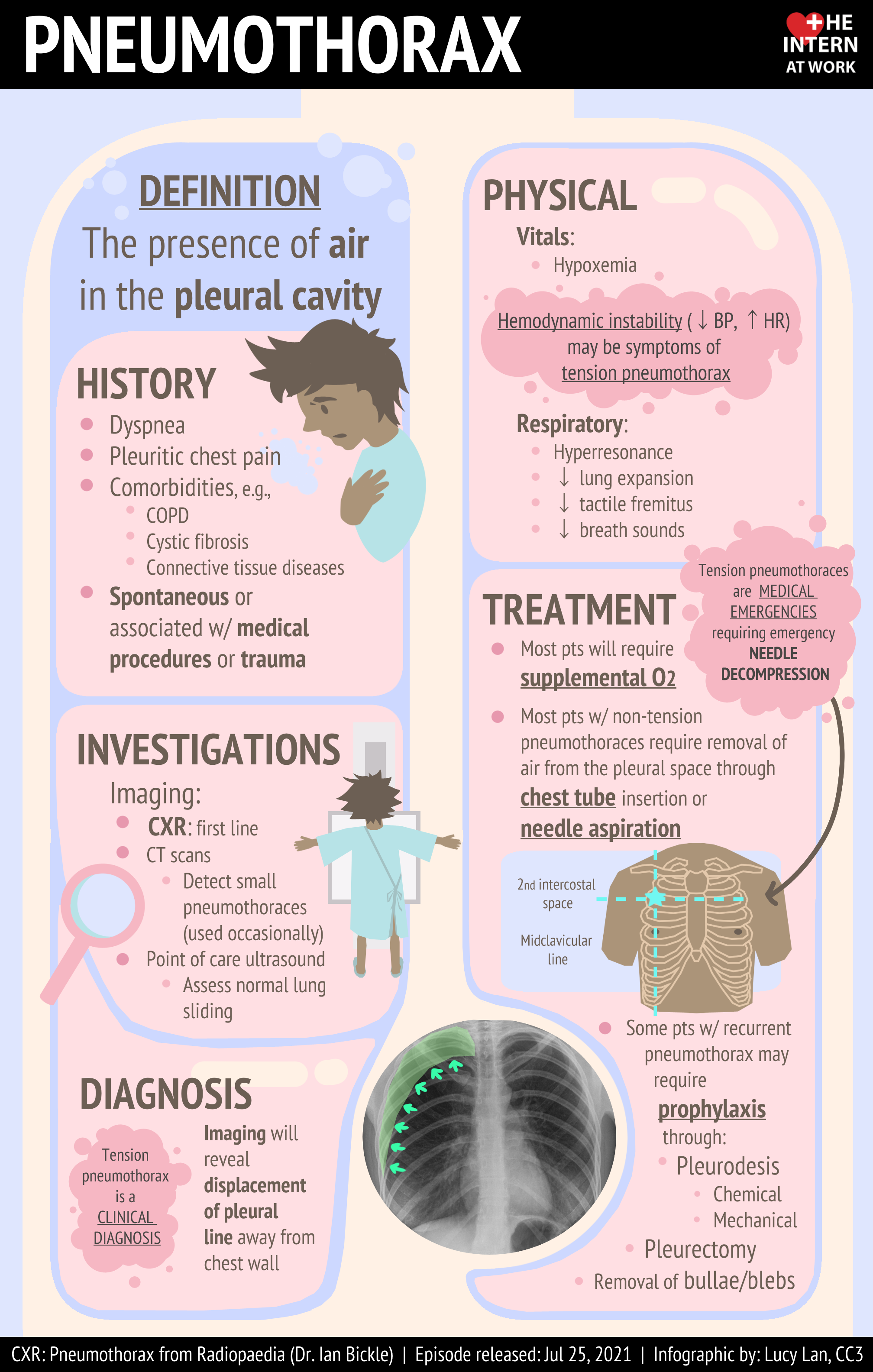
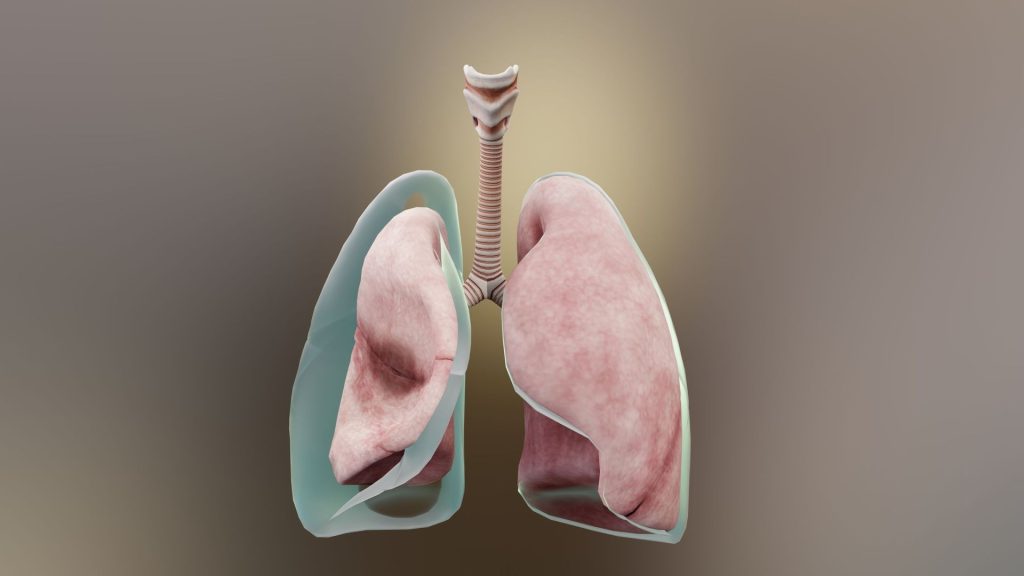
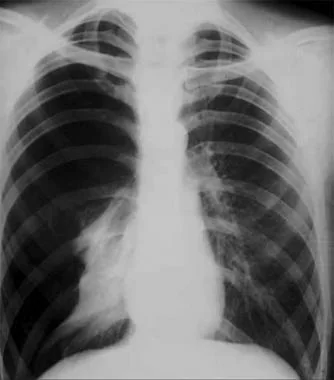
Pneumothorax is defined as the presence of air or gas in the pleural cavity (ie, the potential space between the visceral and parietal pleura of the lung), which can impair oxygenation and/or ventilation. The clinical results are dependent on the degree of collapse of the lung on the affected side. If the pneumothorax is significant, it can cause a shift of the mediastinum and compromise hemodynamic stability. Air can enter the intrapleural space through a communication from the chest wall (ie, trauma) or through the lung parenchyma across the visceral pleura. See the image below.
Signs and symptoms
The presentation of patients with pneumothorax varies depending on the following types of pneumothorax and ranges from completely asymptomatic to life-threatening respiratory distress:
- Spontaneous pneumothorax: No clinical signs or symptoms in primary spontaneous pneumothorax until a bleb ruptures and causes pneumothorax; typically, the result is acute onset of chest pain and shortness of breath, particularly with secondary spontaneous pneumothoraces
- Iatrogenic pneumothorax: Symptoms similar to those of spontaneous pneumothorax, depending on patient’s age, presence of underlying lung disease, and extent of pneumothorax
- Tension pneumothorax: Hypotension, hypoxia, chest pain, dyspnea
- Catamenial pneumothorax: Women aged 30-40 years with onset of symptoms within 48 hours of menstruation, right-sided pneumothorax, and recurrence
- Pneumomediastinum: Must be differentiated from spontaneous pneumothorax; patients may or may not have symptoms of chest pain, persistent cough, sore throat, dysphagia, shortness of breath, or nausea/vomiting
Diagnosis
History and physical examination remain the keys to making the diagnosis of pneumothorax. Examination of patients with this condition may reveal diaphoresis and cyanosis (in the case of tension pneumothorax). Affected patients may also reveal altered mental status changes, including decreased alertness and/or consciousness (a rare finding).
Findings on lung auscultation vary depending on the extent of the pneumothorax. Respiratory findings may include the following:
- Respiratory distress (considered a universal finding) or respiratory arrest
- Tachypnea (or bradypnea as a preterminal event)
- Asymmetric lung expansion: Mediastinal and tracheal shift to contralateral side (large tension pneumothorax)
- Distant or absent breath sounds: Unilaterally decreased/absent lung sounds common, but decreased air entry may be absent even in advanced state of pneumothorax
- Minimal lung sounds transmitted from unaffected hemithorax with auscultation at midaxillary line
- Hyperresonance on percussion: Rare finding; may be absent even in an advanced state
- Decreased tactile fremitus
- Adventitious lung sounds: Ipsilateral crackles, wheezes
Cardiovascular findings may include the following:
- Tachycardia: Most common finding; if heart rate is faster than 135 beats/min, tension pneumothorax likely
- Pulsus paradoxus
- Hypotension: Inconsistently present finding; although typically considered a key sign of tension pneumothorax, hypotension can be delayed until its appearance immediately precedes cardiovascular collapse
- Jugular venous distention: Generally seen in tension pneumothorax; may be absent if hypotension is severe
- Cardiac apical displacement: Rare finding
Common findings among the types of pneumothoraces include the following:
- Spontaneous and iatrogenic pneumothorax: Tachycardia most common finding; tachypnea and hypoxia may be present
- Tension pneumothorax: Variable findings; respiratory distress and chest pain; tachycardia; ipsilateral air entry on auscultation; breath sounds absent on affected hemithorax; trachea may deviate from affected side; thorax may be hyperresonant; jugular venous distention and/or abdominal distention may be present
- Pneumomediastinum: Variable or absent findings; subcutaneous emphysema is the most consistent sign; Hamman sign—a precordial crunching noise synchronous with the heartbeat and often accentuated during expiration—has a variable rate of occurrence, with one series reporting 10%
Lab and imaging studies
Although laboratory and imaging studies help determine a diagnosis, tension pneumothorax primarily is a clinical diagnosis based on patient presentation. Suspicion of tension pneumothorax, especially in late stages, mandates immediate treatment and does not require potentially prolonged diagnostic studies.
Arterial blood gas (ABG) studies measure the degrees of acidemia, hypercarbia, and hypoxemia, the occurrence of which depends on the extent of cardiopulmonary compromise at the time of collection. ABG analysis does not replace physical diagnosis, nor should treatment be delayed while awaiting results if symptomatic pneumothorax is suspected. However, ABG analysis may be useful in evaluating hypoxia and hypercarbia and respiratory acidosis.
When pneumothorax is suspected, confirmation by chest radiography affords additional information beyond confirmation, such as the extent of pneumothorax, potential causes, a baseline study from which to go forward, and assistance with the therapeutic plan.
The following radiologic studies may be used to evaluate suspected pneumothorax:
- Chest radiography: Anteroposterior and/or lateral decubitus films
- Contrast-enhanced esophagography: If emesis/retching is the precipitating event
- Chest computed tomography scanning: Most reliable imaging study for diagnosis of pneumothorax but not recommended for routine use in pneumothorax
- Chest ultrasonography
Management
Although there is general agreement on the management of pneumothorax, a full consensus about management of initial or recurrent pneumothorax does not exist. Rather, many clinicians use a risk stratification framework as well as other approaches for choosing among options to restore lung volume and an air-free pleural space and to prevent recurrences. [1]
The range of medical therapeutic options for pneumothorax includes the following:
- Watchful waiting, with or without supplemental oxygen
- Simple aspiration
- Tube drainage, with or without medical pleurodesis
Surgery
If the patient has had repeated episodes of pneumothorax or if the lung remains unexpanded after 5 days with a chest tube in place, operative therapy such as the following may be necessary:
- Thoracoscopy: Video-assisted thoracoscopic surgery (VATS)
- Electrocautery: Pleurodesis or sclerotherapy
- Laser treatment
- Resection of blebs or pleura
- Open thoracotomy
Pharmacotherapy
The following medications may be used to aid in the management of patients with pneumothorax:
- Local anesthetics (eg, lidocaine hydrochloride)
- Opioid anesthetics (eg, fentanyl citrate, morphine)
- Benzodiazepines (eg, midazolam, lorazepam)
- Antibiotics (eg, doxycycline, cefazolin)
Background
Pneumothorax is defined as the presence of air or gas in the pleural cavity (ie, the potential space between the visceral and parietal pleura of the lung). The clinical results are dependent on the degree of collapse of the lung on the affected side. Pneumothorax can impair oxygenation and/or ventilation. If the pneumothorax is significant, it can cause a shift of the mediastinum and compromise hemodynamic stability. Air can enter the intrapleural space through a communication from the chest wall (ie, trauma) or through the lung parenchyma across the visceral pleura.
Among the topics this article will discuss are several areas of new information in the medical literature: (1) studies comparing aspiration and tube drainage for treatment of primary spontaneous pneumothorax (PSP), (2) long-term follow-up of surgical treatment of pneumothorax, (3) assessment of the impact of pleurodesis on transplantation outcomes in patients with lymphangiomyomatosis, (4) demonstrated utility of ultrasonography (US) in the bedside diagnosis of iatrogenic pneumothorax, and (5) inability of US to distinguish between intrapulmonary bullae and pneumothorax.
Primary and secondary spontaneous pneumothorax
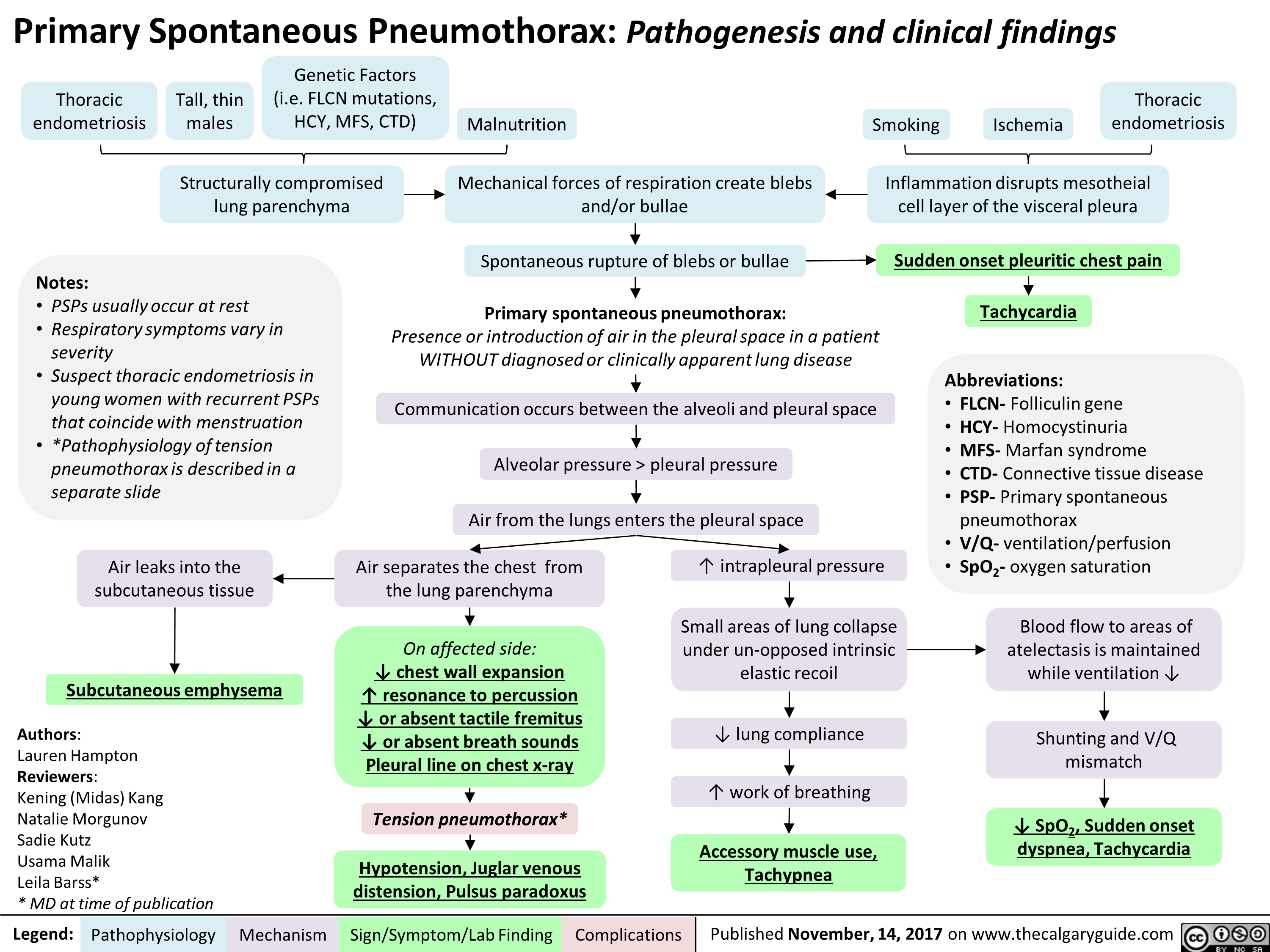
Spontaneous pneumothorax is a commonly encountered problem with approaches to treatment that vary from observation to aggressive intervention. PSP occurs in people without underlying lung disease and in the absence of an inciting event (see the images below). Air enters into the intrapleural space without preceding trauma and without an underlying history of clinical lung disease. However, many patients whose condition is labeled as PSP have subclinical lung disease (eg, pleural blebs) that can be detected with computed tomography (CT). Patients are typically aged 18-40 years, tall, and thin; often, they are smokers.
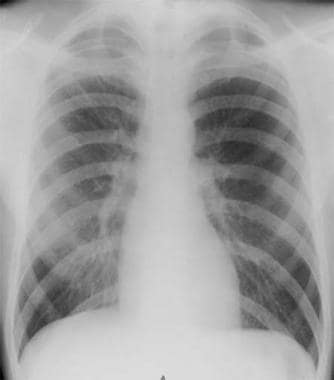
Radiograph of patient with small spontaneous primary pneumothorax.
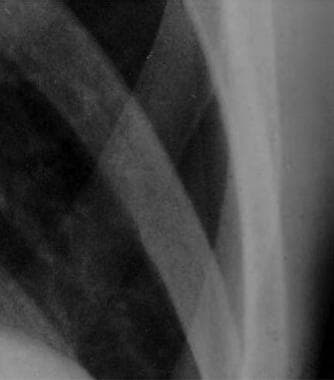
Close radiographic view of patient with small spontaneous primary pneumothorax (same patient as in previous image).
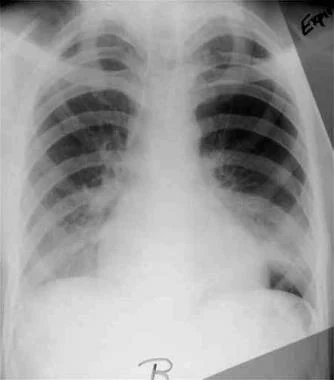
Expiratory radiograph of patient with small spontaneous primary pneumothorax (same patient as in previous images).
Secondary spontaneous pneumothorax (SSP) occurs in people with a wide variety of parenchymal lung diseases. These individuals have underlying pulmonary pathology that alters normal lung structure (see the image below). Air enters the pleural space via distended, damaged, or compromised alveoli. The presentation of these patients may include more serious clinical symptoms and sequelae due to comorbid conditions.
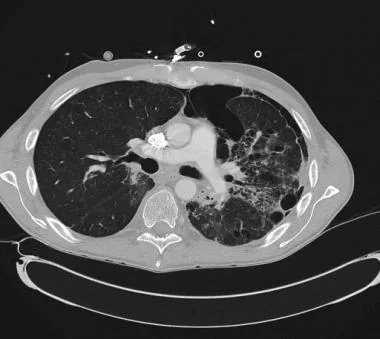
CT scan demonstrating secondary spontaneous pneumothorax (SSP) from radiation/chemotherapy for lymphoma.
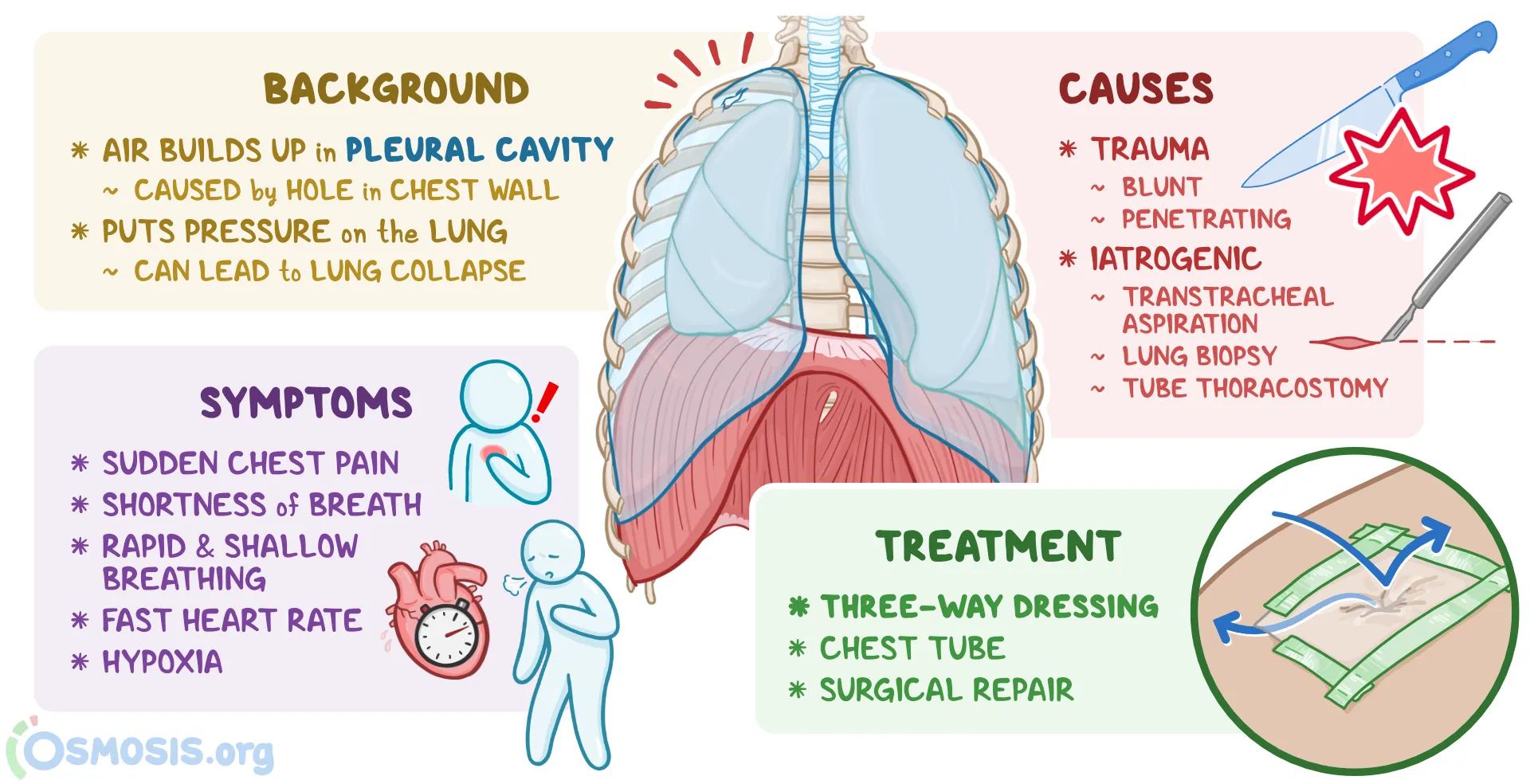
Iatrogenic and traumatic pneumothorax
Iatrogenic pneumothorax is a traumatic pneumothorax that results from injury to the pleura, with air introduced into the pleural space secondary to diagnostic or therapeutic medical intervention (see the following image). Half a century ago, iatrogenic pneumothorax was predominantly the result of deliberate injection of air into the pleural space for the treatment of tuberculosis (TB). The terminology evolved to the preference for “induced” or “artificial” pneumothorax to indicate pulmonary TB treatment, before arriving at the current classification. Pulmonary TB remains a significant cause of secondary pneumothorax.
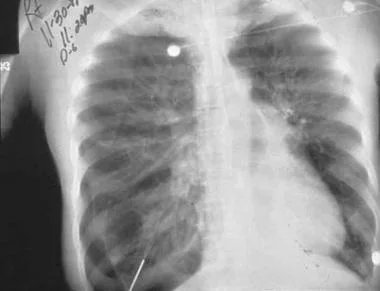
Radiograph of older man who was admitted to ICU postoperatively. Note right-side pneumothorax induced by incorrectly positioned small-bowel feeding tube in right-side bronchial tree. Marked depression of right hemidiaphragm is noted, and mediastinal shift is to left, suggestive of tension pneumothorax. Endotracheal tube is in good position.
Traumatic pneumothorax results from blunt trauma or penetrating trauma that disrupts the parietal or visceral pleura (see the images below). Management steps for traumatic pneumothoraces are similar to those for other, nontraumatic causes. If hemodynamic or respiratory status is compromised or an open (communicating to the atmosphere) and/or hemothorax are also present, tube thoracostomy is performed to evacuate air and allow reexpansion of the lung. There is a subset of traumatic pneumothoraces classified as occult; that is, they cannot be seen on chest radiographs but can be seen on CT scans. In general, these can be observed and treated if they become symptomatic.
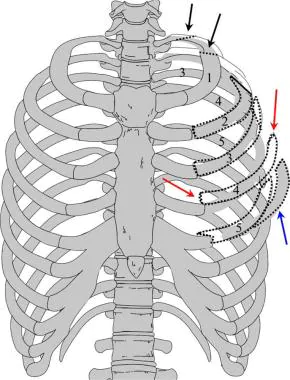
Illustration depicting multiple fractures of left upper chest wall. First rib is often fractured posteriorly (black arrows). If multiple rib fractures occur along midlateral (red arrows) or anterior chest wall (blue arrows), flail chest (dotted black lines) may result, which may result in pneumothorax.
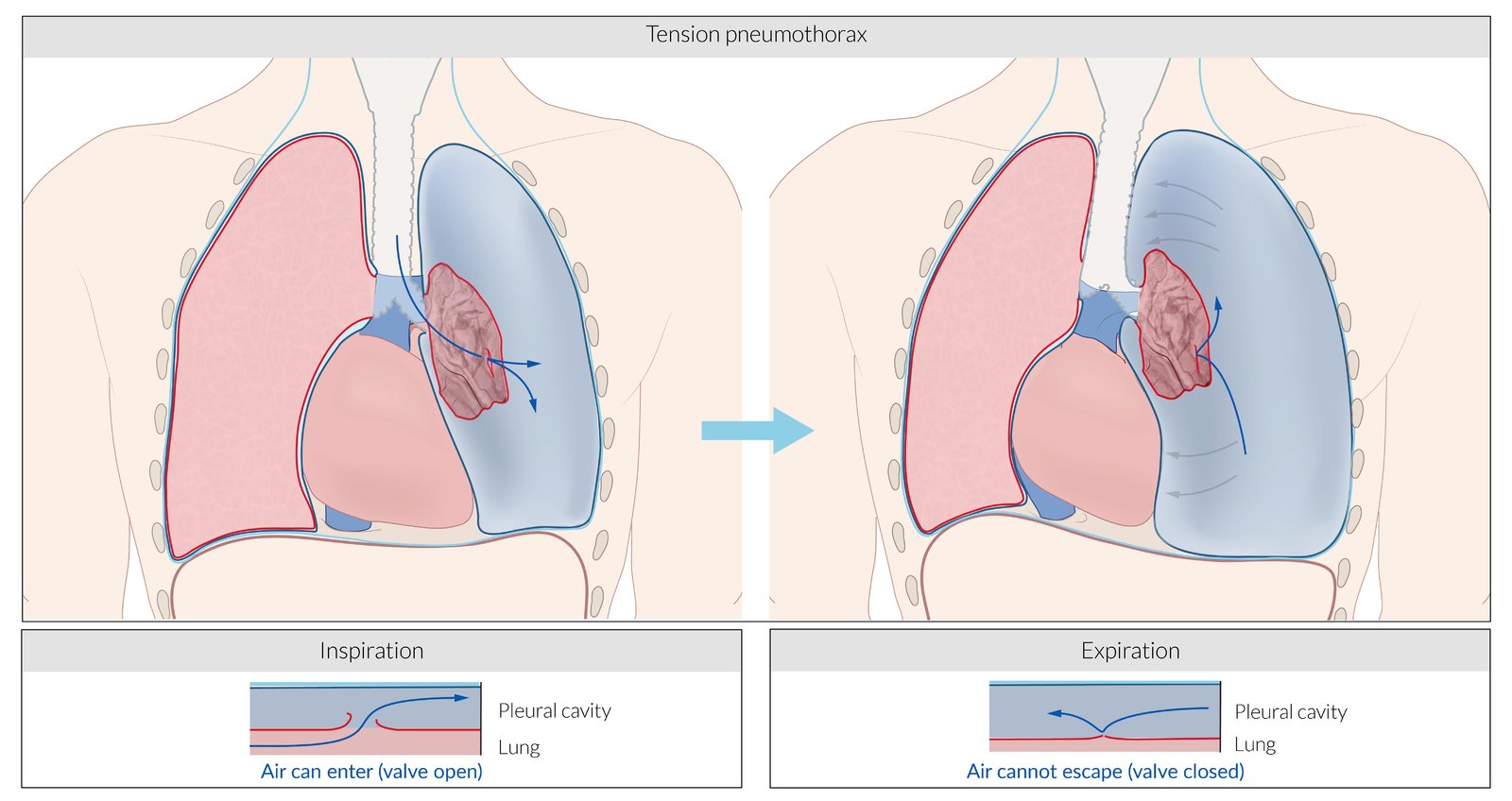
Tension pneumothorax
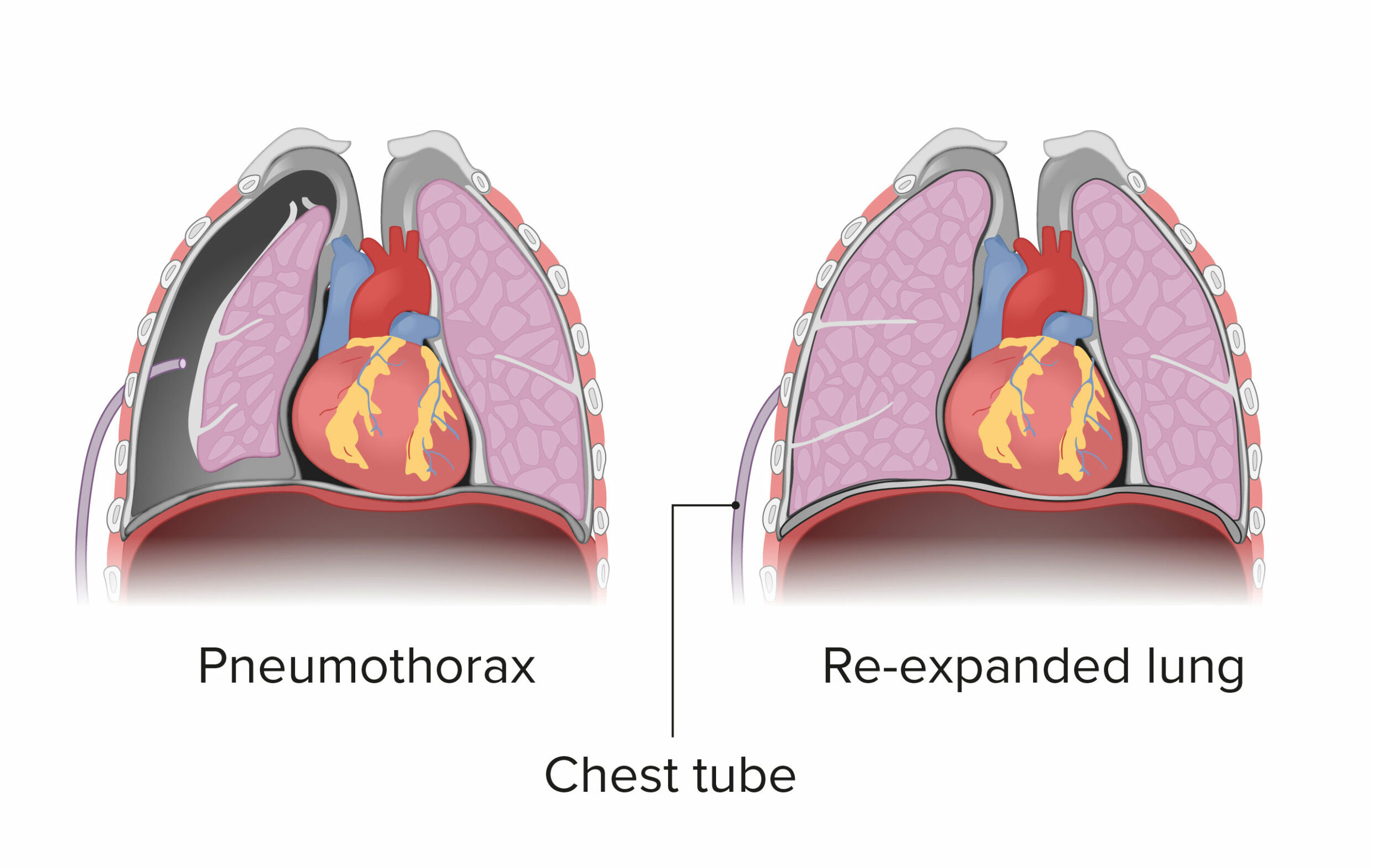
Tension pneumothorax is a life-threatening condition that develops when air is trapped in the pleural cavity under positive pressure, displacing mediastinal structures and compromising cardiopulmonary function. Prompt recognition is life-saving, both outside the hospital and in a modern intensive care unit (ICU). Because tension pneumothorax occurs infrequently and has a potentially devastating outcome, a high index of suspicion and knowledge of basic emergency thoracic decompression procedures are important for all healthcare personnel. Immediate decompression of the thorax is mandatory when tension pneumothorax is suspected and should not be delayed for radiographic confirmation. (See the image below.)
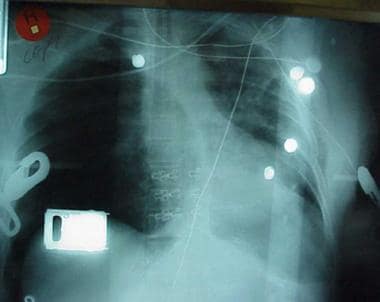
Chest radiograph shows two abnormalities: (1) tension pneumothorax and (2) potentially life-saving intervention delayed during wait for x-ray results. Tension pneumothorax is clinical diagnosis requiring emergency needle decompression, and therapy should never be delayed for x-ray confirmation.
Pneumomediastinum
Pneumomediastinum is the presence of gas in the mediastinal tissues occurring spontaneously or following procedures or trauma (see the following images). A pneumothorax may occur secondary to pneumomediastinum.
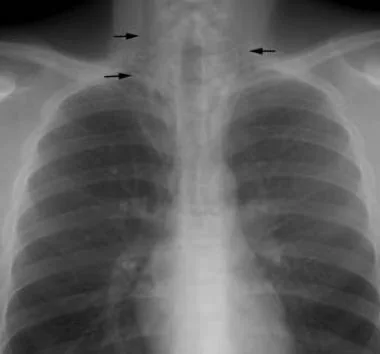
Pneumomediastinum from barotrauma may result in tension pneumothorax and obstructive shock.
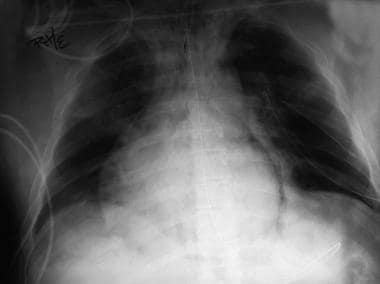
Chest radiograph shows pneumomediastinum (radiolucency noted around left heart border) in patient who had respiratory and circulatory arrest in emergency department after experiencing multiple episodes of vomiting and rigid abdomen. Patient was taken immediately to operating room, where large rupture of esophagus was repaired.
Anatomy
The inner surface of the thoracic cage (parietal pleura) is contiguous with the outer surface of the lung (visceral pleura); this space contains a small amount of lubricating fluid and is normally under negative pressure compared to the alveoli. Determinants of pleural pressure are the opposing recoil forces of the lung and chest wall.
Pathophysiology
Spontaneous pneumothorax
Spontaneous pneumothorax in most patients occurs from the rupture of blebs and bullae. Although PSP is defined as occurring in patients without underlying pulmonary disease, these patients have asymptomatic blebs and bullae detected on CT scans or during thoracotomy. PSP is typically observed in tall, young people without parenchymal lung disease and is thought to be related to increased shear forces in the apex.
Although PSP is associated with the presence of apical pleural blebs, the exact anatomic site of air leakage is often uncertain. Fluorescein-enhanced autofluorescence thoracoscopy (FEAT) is a newer method of examining the site of air leak in PSP. FEAT-positive lesions can be detected that appear normal when viewed under normal white-light thoracoscopy.
In normal respiration, the pleural space has a negative pressure. As the chest wall expands outward, the surface tension between the parietal and visceral pleura expands the lung outward. The lung tissue intrinsically has an elastic recoil, tending to collapse inwards. If the pleural space is invaded by gas from a ruptured bleb, the lung collapses until equilibrium is achieved or the rupture is sealed. As the pneumothorax enlarges, the lung becomes smaller. The main physiologic consequence of this process is a decrease in vital capacity and partial pressure of oxygen.
Lung inflammation and oxidative stress are hypothesized to be important to the pathogenesis of PSP. Current smokers, at increased risk for PSP, have increased numbers of inflammatory cells in the small airways. Bronchoalveolar lavage (BAL) studies in patients with PSP reveal that the degree of inflammation correlates with the extent of emphysematouslike changes (ELCs). One hypothesis is that ELCs result from degradation of lung tissue due to imbalances of enzymes and antioxidants released by innate immune cells. In one study, erythrocyte superoxide dismutase activity was significantly lower and plasma malondialdehyde levels higher in patients with PSP than in normal control subjects.
A growing body of evidence suggests that genetic factors may be important in the pathogenesis of many cases of PSP. Familial clustering of this condition has been reported. Genetic disorders that have been linked to PSP include Marfan syndrome, homocystinuria, and Birt-Hogg-Dube (BHD) syndrome.
BHD syndrome is an autosomal dominant disorder that is characterized by benign skin tumors (hair follicle hamartomas), renal and colon cancer, and spontaneous pneumothorax. Spontaneous pneumothorax occurs in about 22% of patients with this syndrome. The gene responsible is a tumor suppressor gene located on band 17p11.2. The gene encoding folliculin (FLCN) is thought to be the cause. Multiple mutations have been found, and phenotypic variation is recognized. In one study, eight patients without skin or renal involvement had lung cysts and spontaneous pneumothorax. A germline mutation to this gene has been found in five patients, and genetic testing is now available.
Tension pneumothorax
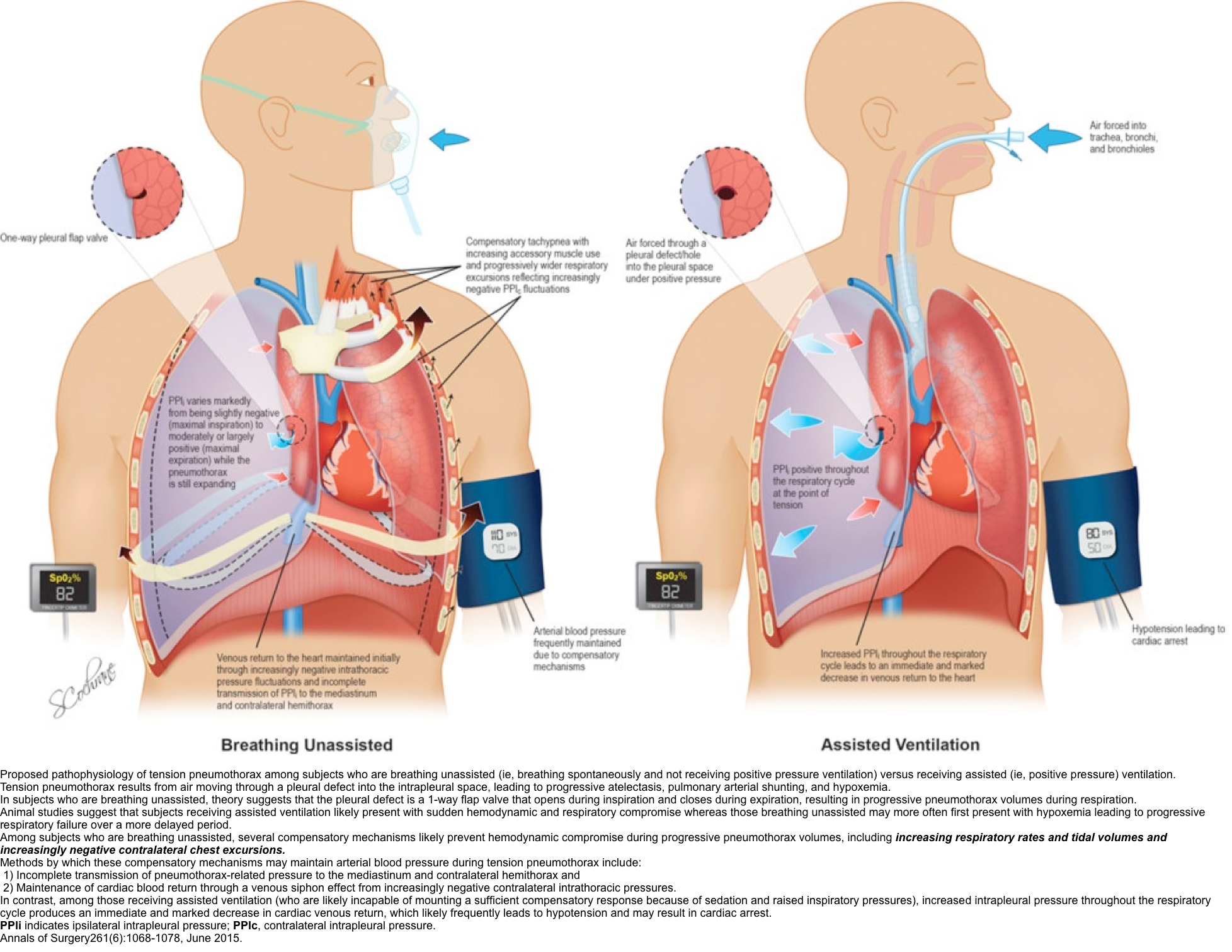
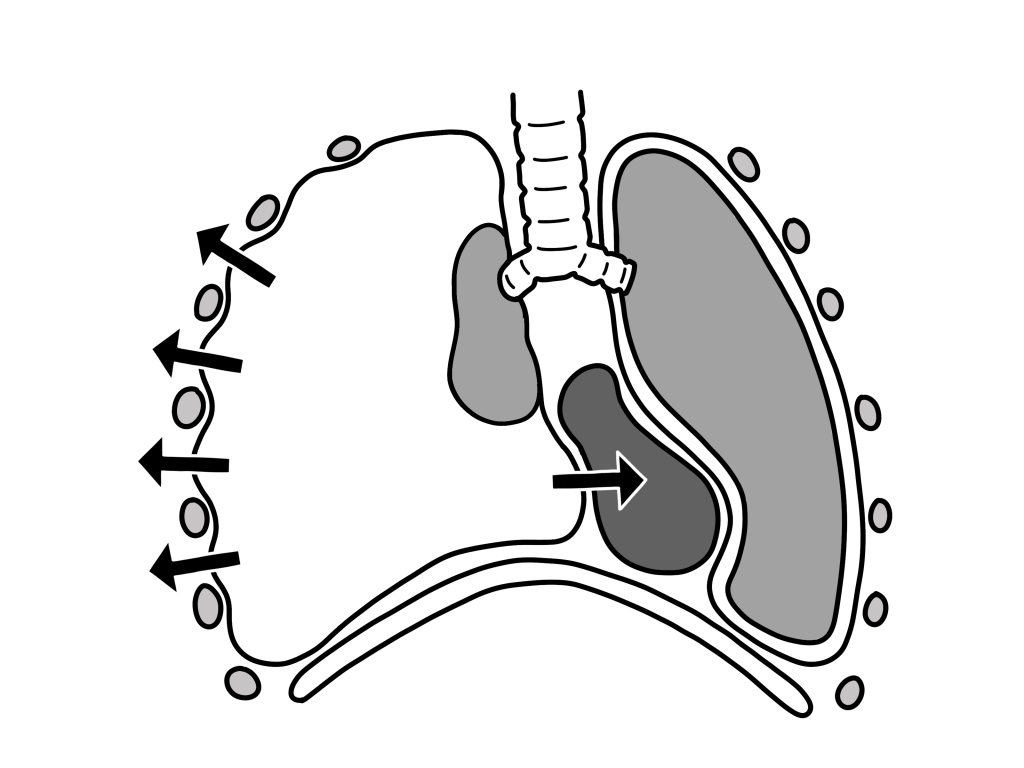
Tension pneumothorax occurs anytime a disruption involves the visceral pleura, parietal pleura, or the tracheobronchial tree. This condition develops when injured tissue forms a one-way valve, allowing air inflow with inhalation into the pleural space and prohibiting air outflow. The volume of this nonabsorbable intrapleural air increases with each inspiration because of the one-way valve effect. As a result, pressure rises within the affected hemithorax. In addition to this mechanism, the positive pressure used with mechanical ventilation can cause air trapping.
As pressure increases, the ipsilateral lung collapses and causes hypoxia. Further pressure increases cause the mediastinum to shift toward the contralateral side, impinging on and compressing the contralateral lung and impairing venous return to the right atrium. Hypoxia results as the collapsed lung on the affected side and the compressed lung on the contralateral side compromise gas exchange. This hypoxia and decreased venous return caused by compression of the relatively thin walls of the atria impair cardiac function. Kinking of the inferior vena cava is thought to be the initial event restricting blood to the heart. It is most evident in trauma patients who are hypovolemic with reduced venous blood return.
Arising from numerous causes, this condition rapidly progresses to respiratory insufficiency, cardiovascular collapse, and, ultimately, death if unrecognized and untreated.
Pneumomediastinum
With pneumomediastinum, excessive intra-alveolar pressures lead to rupture of alveoli bordering the mediastinum. Air escapes into the surrounding connective tissue and dissects further into the mediastinum. Esophageal trauma or elevated airway pressures may also allow air to dissect into the mediastinum. Air may then travel superiorly into the visceral, retropharyngeal, and subcutaneous spaces of the neck. From the neck, the subcutaneous compartment is continuous throughout the body; thus, air can diffuse widely.
Mediastinal air can also pass inferiorly into the retroperitoneum and other extraperitoneal compartments. If the mediastinal pressure rises abruptly or if decompression is not sufficient, the mediastinal parietal pleura may rupture and cause a pneumothorax (in 10-18% of patients).
A wide variety of disease states and circumstances may result in a pneumothorax.
Primary and secondary spontaneous pneumothorax
Risks factors for PSP include the following:
- Smoking
- Tall, thin stature in a healthy person
- Marfan syndrome
- Pregnancy
- Familial pneumothorax
Blebs and bullae (sometimes referred to as ELCs) are related to the occurrence of PSP. Thoracic CT scans of patients with PSP shows ipsilateral ELC in 89% and contralateral changes in 80%, compared with a rate of 20% among control subjects matched for age and smoking. Nonsmokers with PSP had CT scan ELC abnormalities of 80% compared with a rate of 0% among nonsmoker controls without PSP.
Although patients with PSP do not have overt parenchymal disease, this condition is heavily associated with smoking—80-90% of PSP cases occur in smokers or former smokers, and the relative risk of PSP increases as the number of cigarettes smoked per day increases; that is, the risk of PSP is related to the intensity of smoking, with 102 times higher incidence rates in males who smoke heavily (ie, >22 cigarettes/d), compared with a sevenfold increase in males who smoke lightly (1-12 cigarettes/d). This incremental risk with increasing number of cigarettes smoked per day is much more pronounced in female smokers.
Typical PSP patients also tend to have a tall and thin body habitus. Whether height affects development of subpleural blebs or whether more negative apical pleural pressures cause preexisting blebs to rupture is unclear.
Pregnancy is an unrecognized risk factor, as suggested by a 10-year retrospective series in which five of 250 spontaneous pneumothorax cases were in pregnant women. The cases were all managed successfully with simple aspiration or video-assisted thoracoscopic surgery (VATS), and no harm occurred to mother or fetus.
Other associations with pneumothorax include increased intrathoracic pressure with the Valsalva maneuver, though contrary to popular belief, most spontaneous pneumothoraces occur while the patient is at rest. Changes in atmospheric pressure, proximity to loud music, and low-frequency noises are other reported factors.
Familial associations have been noted in more than 10% of patients. Some are due to rare connective tissue diseases, but mutations in the gene encoding folliculin (FLCN) have been described. These patients may represent an incomplete penetrance of an autosomal dominant genetic disorder. BHD syndrome is characterized by benign skin growths, pulmonary cysts, and renal cancers and is caused by mutations in the FLCN gene.
In one family study, nine ascertained cases of spontaneous pneumothorax were reported among 54 members. A review of the literature summarized 61 reports of familial spontaneous pneumothorax among 22 families. Up to 10% patients with spontaneous pneumothorax report a positive family history.
Although rare, spontaneous pneumothorax occurring bilaterally and progressing to tension pneumothorax has been documented.
Diseases and conditions associated with SSP include the following:
- Chronic obstructive pulmonary disease (COPD) or emphysema – Increased pulmonary pressure due to coughing with a bronchial plug of mucus or phlegm bronchial plug may play a role.
- Asthma
- Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) with PCP infection
- Necrotizing pneumonia
- Tuberculosis
- Sarcoidosis
- Cystic fibrosis
- Bronchogenic carcinoma or metastatic malignancy
- Idiopathic pulmonary fibrosis
- Inhalational and intravenous drug use (eg, marijuana, cocaine)
- Interstitial lung diseases associated with connective tissue diseases
- Lymphangioleiomyomatosis (LAM)
- Langerhans cell histiocytosis
- Severe acute respiratory syndrome (SARS) – A reported 1.7% of SARS patients developed spontaneous pneumothorax.
- Thoracic endometriosis and catamenial pneumothorax
- Collagen vascular disease, including Marfan syndrome
SSPs occur in the presence of lung disease, primarily in the presence of COPD. Other diseases that may be present when SSPs occur include tuberculosis, sarcoidosis, cystic fibrosis, malignancy, and idiopathic pulmonary fibrosis.
Pneumocystis jiroveci pneumonia (previously known as Pneumocystis cariniipneumonia [PCP]) was a common cause of SSP in patients with AIDS during the last decade. In fact, 77% of AIDS patients with spontaneous pneumothorax had thin-walled cavities, cysts, and pneumothorax from PCP infection. With the advent of highly active antiretroviral therapy (HAART) and widespread use of trimethoprim-sulfamethoxazole (TMP-SMZ) prophylaxis, the incidence of PCP and associated SSP has significantly declined.
PCP in other immunocompromised patients is seen only when TMP-SMZ prophylaxis is withdrawn prematurely. For practical purposes, if the immunocompromised patient has been taking TMP-SMZ prophylaxis reliably, PCP is reasonably excluded from the differential diagnosis and should not be a causative factor for SSP.
In cystic fibrosis, up to 18.9% of patients have been reported to develop spontaneous pneumothoraces, and they have a high incidence of recurrence on the same side after conservative management (50%) or intercostal drainage (55.2%). The risk of SSP in these patients increases with Burkholderia cepacia or Pseudomonas infections and allergic bronchopulmonary aspergillosis (ABPA). Pleurodesis increases the risk of bleeding associated with lung transplantation but is not an absolute contraindication.
Many different types of malignancies are known to present with a pneumothorax, especially sarcomas, but also genitourinary cancers and primary lung cancer; thus, pneumothorax in a patient with malignancy should prompt a look for metastatic disease. Chemotherapeutic agents, at times, can also induce SSP.
Interstitial lung diseases are associated with connective-tissue diseases. Ankylosing spondylitis may be noted when apical fibrosis is present; in fact, the typically low incidence of spontaneous pneumothorax in patients with ankylosing spondylitis (0.29%) increases 45-fold (to 13%) when apical fibrotic disease exists.
LAM may present with spontaneous pneumothorax. This disease is characterized by thin-walled cysts in women of childbearing age. Respiratory failure may lead to a need for lung transplantation, and previous pleurodesis is no longer an absolute contraindication for lung transplantation.
Thoracic endometriosis is a rare cause of recurrent pneumothorax (catamenial pneumothorax) in women that is thought to arise from endometriosis reaching the chest wall across the diaphragm (ie, its etiology may be primarily related to associated diaphragmatic defects). In a case series of 229 patients, catamenial pneumothorax caused by thoracic endometriosis was localized to the visceral pleura in 52% of patients and to the diaphragm in 39% of patients. Before recurrence, this condition may be initially diagnosed as PSP.
Iatrogenic and traumatic pneumothorax
Causes of iatrogenic pneumothorax include the following:
- Transthoracic needle aspiration biopsy of pulmonary nodules (most common cause: 32-37% of cases)
- Transbronchial or pleural biopsy
- Thoracentesis
- Central venous catheter (CVC) insertion, usually subclavian or internal jugular
- Intercostal nerve block
- Tracheostomy
- Cardiopulmonary resuscitation (CPR) – Consider the possibility of a pneumothorax if ventilation becomes progressively more difficult.
- Acute respiratory distress syndrome ( ARDS) and positive-pressure ventilation in the ICU – High peak airway pressures can translate into barotrauma in up to 3% of patients on a ventilator and up to 5% of patients with ARDS.
- Nasogastric feeding tube placement
Iatrogenic pneumothorax is a complication of medical or surgical procedures. It most commonly results from transthoracic needle aspiration. Other procedures commonly causing iatrogenic pneumothorax are therapeutic thoracentesis, pleural biopsy, CVC insertion, transbronchial biopsy, positive-pressure ventilation (PPV), and inadvertent intubation of the right mainstem bronchus. Therapeutic thoracentesis is complicated by pneumothorax 30% of the time when performed by inexperienced operators, in contrast to only 4% of the time when performed by experienced clinicians.
The routine use of US during diagnostic thoracentesis is associated with lower rates of pneumothorax (4.9% vs 10.3%) and need for tube thoracostomy (0.7% vs 4.1%). Similarly, in patients who are mechanically ventilated, thoracocentesis guided by bedside US without radiology support results in a relatively lower rate of pneumothorax.
Causes of traumatic pneumothorax include the following:
- Trauma – Penetrating and nonpenetrating injury
- Rib fracture
- High-risk occupation (eg, diving, flying)
Traumatic pneumothoraces can result from both penetrating and nonpenetrating lung injuries. Complications include hemopneumothorax and bronchopleural fistula. Traumatic pneumothoraces often can create a one-way valve in the pleural space (only letting in air without escape) and can lead to a tension pneumothorax.
Tension pneumothorax
The most common etiologies of tension pneumothorax are either iatrogenic or related to trauma, such as the following:
- Blunt or penetrating trauma – Disruption of either the visceral or parietal pleura occurs and is often associated with rib fractures, though rib fractures are not necessary for tension pneumothorax to occur.
- Barotrauma secondary to PPV, especially when high amounts of positive end-expiratory pressure (PEEP) are used
- Pneumoperitoneum
- Fiberoptic bronchoscopy with closed lung biopsy
- Markedly displaced thoracic spine fractures
- Acupuncture
- Preexisting Bochdalek hernia with trauma
- Colonoscopy and gastroscopy have been implicated in case reports.
- Percutaneous tracheostomy
- Conversion of idiopathic, spontaneous, simple pneumothorax to tension pneumothorax
- Unsuccessful attempts to convert an open pneumothorax to a simple pneumothorax in which the occlusive dressing functions as a one-way valve
Tension pneumothorax occurs commonly in the ICU setting in patients who are receiving PPV, and practitioners must always consider this when changes in respiratory or hemodynamic status occur. Infants requiring ventilatory assistance and those with meconium aspiration have a particularly high risk for tension pneumothorax. Aspirated meconium may serve as a one-way valve and produce a tension pneumothorax.
Any penetrating wound that produces an abnormal passageway for gas exchange into the pleural spaces and that results in air trapping may produce a tension pneumothorax. Blunt trauma, with or without associated rib fractures, and incidents such as unrestrained head-on motor vehicle accidents, falls, and altercations involving laterally directed blows may also cause tension pneumothoraces.
Significant chest injuries carry an estimated 10-50% risk of associated pneumothorax; in about 50% of these cases, the pneumothorax may not be seen on standard radiographs and are therefore deemed occult. In one study, 12% of patients with asymptomatic chest stab wounds had a delayed pneumothorax or hemothorax. McPherson et al analyzed data from the Vietnam Wound Data and Munitions Effectiveness Team study and determined that tension pneumothorax was the cause of death in 3-4% of fatally wounded combat casualties.
Acupuncture is a traditional Chinese medicine technique used worldwide by alternative medical practitioners. Acupuncture’s most frequently reported serious complication is pneumothorax; in one Japanese report of 55,291 acupuncture treatments, an approximate incidence of 1 pneumothorax in 5000 cases was documented.
Pneumomediastinum
The following factors may result in pneumomediastinum:
- Acute generation of high intrathoracic pressures (often as a result of inhalational drug use, such smoking marijuana or inhalation of cocaine)
- Asthma
- Respiratory tract infection
- Parturition
- Emesis
- Severe cough
- Mechanical ventilation
- Trauma or surgical disruption of the oropharyngeal, esophageal, or respiratory mucosa
- Athletic competition
Epidemiology
Primary, secondary, and recurring spontaneous pneumothorax
It is likely that the incidence for spontaneous pneumothorax is underestimated. Up to 10% of patients may be asymptomatic, and others with mild symptoms may not present to a medical provider.
PSP occurs in people aged 20-30 years, with a peak incidence is in the early 20s; it is rarely observed in people older than 40 years. The age-adjusted incidence of PSP is 7.4-18 cases per 100,000 persons per year for men and 1.2-6 cases per 100,000 persons per year for women. The male-to-female ratio of age-adjusted rates is 6.2:1.
SSPs occur more frequently in patients aged 60-65 years. The age-adjusted incidence of SSP is 6.3 cases per 100,000 persons per year for men and 2.0 cases per 100,000 persons per year for women. The male-to-female ratio of age-adjusted rates is 3.2:1. COPD is a common cause of secondary spontaneous pneumothorax that carries an incidence of 26 cases per 100,000 persons.
Smoking increases the risk of a first spontaneous pneumothorax by more than 20-fold in men and by nearly 10-fold in women compared with risks in nonsmokers. Increased risk of pneumothorax and recurrence appears to rise proportionally with number of cigarettes smoked.
In men, the risk of spontaneous pneumothorax is 102 times higher in heavy smokers than in nonsmokers. Spontaneous pneumothorax most frequently occurs in tall, thin men aged 20-40 years.
Iatrogenic and traumatic pneumothorax
Traumatic and tension pneumothoraces occur more frequently than spontaneous pneumothoraces, and the rate is undoubtedly increasing in United States hospitals as intensive care treatment modalities have become increasingly dependent on PPV, CVC placement, and other causes that potentially induce iatrogenic pneumothorax.
Iatrogenic pneumothorax may cause substantial morbidity and, rarely, death. The incidence of iatrogenic pneumothorax is 5-7 per 10,000 hospital admissions, with thoracic surgery patients excluded because pneumothorax may be a typical outcome following these surgeries.
Pneumothorax occurs in 1-2% of all neonates, with a higher incidence in infants with neonatal respiratory distress syndrome. In one study, 19% of such patients developed a pneumothorax.
Tension pneumothorax
Tension pneumothorax is a complication in approximately 1-2% of the cases of idiopathic spontaneous pneumothorax. Until the late 1800s, TB was a primary cause of pneumothorax development. A 1962 study showed a frequency of pneumothorax of 1.4% in patients with TB.
The actual incidence of tension pneumothorax outside a hospital setting is impossible to determine. Approximately 10-30% of patients transported to level-1 trauma centers in the United States receive prehospital decompressive needle thoracostomies; however, not all of these patients actually have a true tension pneumothorax. Although this occurrence rate may seem high, disregarding the diagnosis would probably result in unnecessary deaths. A review of military deaths from thoracic trauma suggests that as many as 5% of combat casualties with thoracic trauma have tension pneumothorax at the time of death.
The overall incidence of tension pneumothorax in the ICU is unknown. The medical literature provides only glimpses of the frequency. In one report, of 2000 incidents reported to the Australian Incident Monitoring Study (AIMS), 17 involved actual or suspected pneumothoraces, and four of those were diagnosed as tension pneumothorax.
Catamenial pneumothorax
Catamenial pneumothorax is a rare phenomenon that generally occurs in women aged 30-50 years. It frequently begins 1-3 days after menses onset. The risk of thoracic endometriosis cannot be predicted from the site of peritoneal lesions.
Pneumomediastinum
Spontaneous pneumomediastinum generally occurs in young, healthy patients without serious underlying pulmonary disease, mostly in the second to fourth decades of life. A slight predominance of pneumomediastinum exists for males. This condition occurs in approximately 1 case per 10,000 hospital admissions.
Prognosis
Primary, secondary, and recurring spontaneous pneumothorax
Complete resolution of an uncomplicated pneumothorax takes approximately 10 days. PSP is typically benign and often resolves without medical attention. Many affected individuals do not seek medical attention for days after symptoms develop. This trend is important, because the incidence of reexpansion pulmonary edema increases in patients whose chest tubes have been placed 3 days or longer after the pneumothorax occurred.
Recurrences usually strike within the first 6 months to 3 years. The 5-year recurrence rate is 28-32% for PSP and 43% for SSP.
Recurrences are more common among patients who smoke, patients with COPD and patients with AIDS. Predictors of recurrence include pulmonary fibrosis, younger age, and increased height-to-weight ratio. In a retrospective study of 182 consecutive patients with a newly diagnosed first episode of pneumothorax, a higher rate of recurrence was noted in taller patients, thin patients, and patients with SSP.
Patients who underwent bedside chest-tube pleurodesis had cumulative rates of recurrence of 13% at 6 months, 16% at 1 year, and 27% at 3 years, compared with 26%, 33%, and 50%, respectively. The agent used (tetracycline or gentamicin) did not have any significant impact on the recurrence rate.
are also not predictive of recurrence. In a retrospective study of 231 patients with PSP, however, contralateral blebs were more frequently seen by CT in the patients with contralateral recurrence (n = 33; 14%) than in those without a contralateral recurrence. Primary bilateral spontaneous pneumothorax (PBSP) was significantly more common in patients with lower body mass index (BMI) and among smokers.[34] In this series, all patients with contralateral recurrence were treated surgically.
Although some authors view PSP as more of a nuisance than a major health threat, deaths have been reported. SSPs are more often life threatening, depending on the severity of the underlying disease and the size of the pneumothorax (1-17% mortality). In particular, compared with similar patients without pneumothorax, age-matched patients with COPD have a 3.5-fold increase in relative mortality when a spontaneous pneumothorax occurs, and their risk of recurrence rises with each occurrence. One study indicated that 5% of patients with COPD died before a chest tube was placed.
Patients with AIDS also have a high inpatient mortality rate of 25% and a median survival of 3 months after the pneumothorax. These data were derived from an era before highly active antiretroviral therapy (HAART) was available.
Tension pneumothorax
Tension pneumothorax arises from numerous causes and rapidly progresses to respiratory insufficiency, cardiovascular collapse, and, ultimately, death if not recognized and treated. Therefore, if the clinical picture fits a tension pneumothorax, it must be treated on an emergency basis before it results in hemodynamic instability and death.
Pneumomediastinum
Pneumomediastinum is generally a benign, self-limited condition. Malignant pneumomediastinum, or tension pneumomediastinum (unvented mediastinal or pulmonary adventitial air causing pressure so high that circulatory or ventilatory failure occurs), was first described in 1944; however, all patients described in this report had serious comorbid conditions, often related to trauma or in association with Boerhaave syndrome.
The more recent literature has not included reports of fatal outcomes in patients with spontaneous pneumomediastinum in the absence of underlying disease. Mortality is as high as 70% in patients with pneumomediastinum secondary to Boerhaave syndrome, even with surgical intervention. Traumatic mediastinum, though present in as many as 6% of patients, does not portend serious injury.
Patient Education
Two important concerns that clinicians should educate patients with pneumothorax/resolving pneumothorax about are (1) avoidance of travel by air or to remote regions and (2) prohibition of smoking. Patients should also be advised to wear safety belts and passive restraint devices while driving.
Avoidance of travel by air or to remote areas
Patients should not travel by air or travel to remote sites until radiography shows complete resolution. Although commercial air travel causes only minimal change in gas volumes, because of pressurization of the cabin, spontaneous pneumothorax has been described during commercial travel.
Patients with previous spontaneous pneumothoraces are at risk for recurrence and are advised not to dive unless thoracotomy or pleurodesis has been performed. [36] Ascent from deep-sea diving causes gases to expand and can lead to pneumothorax in patients with bullae and blebs.
Smoking cessation
Smoking cessation is strongly advised for all patients. patients should be assessed as to their readiness to quit, should be educated about smoking cessation, and should be provided with pharmacotherapy if ready to quit. Patients indicating a readiness to quit smoking should be directed to their primary care physician or offered referral for cessation management. This may include nicotine replacement and non-nicotine pharmacotherapy (eg, bupropion or varenicline).
History
The presentation of patients with pneumothorax varies depending on the type of pneumothorax.
Spontaneous and iatrogenic pneumothorax
Until a bleb ruptures and causes pneumothorax, no clinical signs or symptoms are present in primary spontaneous pneumothorax (PSP). Young and otherwise healthy patients can tolerate the main physiologic consequences of a decrease in vital capacity and partial pressure of oxygen fairly well, with minimal changes in vital signs and symptoms, but those with underlying lung disease may have respiratory distress.
In one series, acute onset of chest pain and shortness of breath were present in all patients in one series; typically, both symptoms are present in 64-85% of patients. The chest pain is described as severe and/or stabbing, radiates to the ipsilateral shoulder and increases with inspiration (pleuritic).
In PSP, chest often improves over the first 24 hours, even without resolution of the underlying air accumulation. Well-tolerated primary pneumothorax can take 12 weeks to resolve. In secondary spontaneous pneumothorax (SSP), the chest pain is more likely to persist with more significant clinical symptoms.
Shortness of breath/dyspnea in PSP is generally of sudden onset and tends to be more severe with SSP because of decreased lung reserve. Anxiety, cough, and vague presenting symptoms (eg, general malaise, fatigue) are less commonly observed. The most common underlying abnormality in SSP is chronic obstructive pulmonary disease (COPD), and cystic fibrosis carries one of the highest associations, with more than 20% reporting spontaneous pneumothorax.
Despite descriptions of Valsalva maneuvers and increased intrathoracic pressures as inciting factors, spontaneous pneumothorax usually develops at rest. By definition, spontaneous pneumothorax is not associated with trauma or stress. Symptoms of iatrogenic pneumothorax are similar to those of a spontaneous pneumothorax and depend on the age of the patient, the presence of underlying lung disease, and the extent of the pneumothorax.
A history of previous pneumothorax is important, as recurrence is common, with rates reported between 15% and 40%. As many as 15% of recurrences may be on the contralateral side. Secondary pneumothoraces are often more likely to recur, with cystic fibrosis carrying the highest recurrence rates at 68-90%. No study has shown that the number or size of blebs and bullae found in the lung can be used to predict recurrence.
Tension pneumothorax
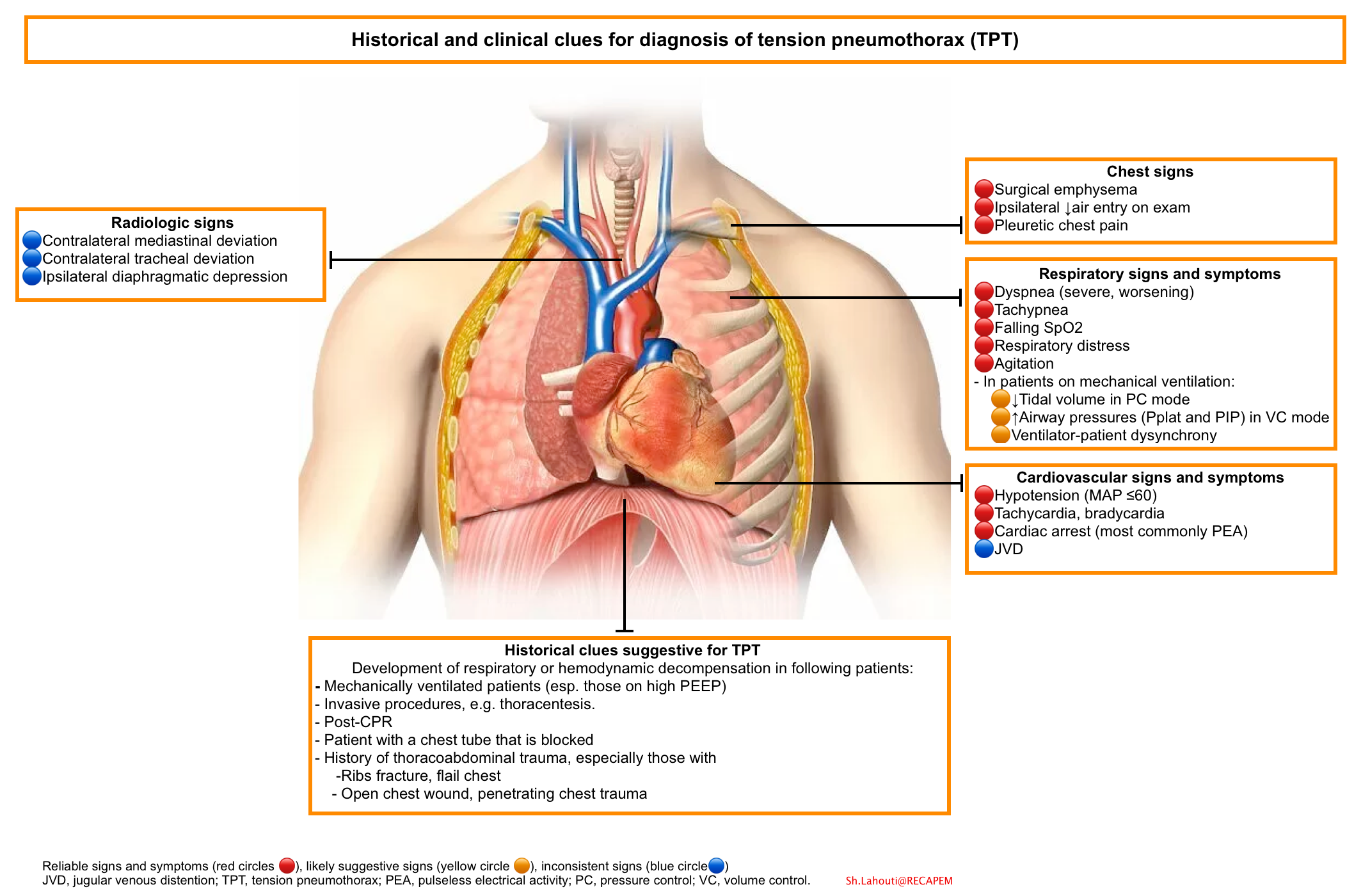
Signs and symptoms of tension pneumothorax are usually more impressive than those seen with a simple pneumothorax, and clinical interpretation of these is crucial for diagnosing and treating the condition. Tension pneumothorax is classically characterized by hypotension and hypoxia. On examination, breath sounds are absent on the affected hemothorax and the trachea deviates away from the affected side. The thorax may also be hyperresonant; jugular venous distention and tachycardia may be present. If on mechanical ventilation, the airway pressure alarms are triggered.
Unlike the obvious patient presentations oftentimes used in medical training courses to describe a tension pneumothorax, actual case reports include descriptions of the diagnosis of the condition being missed or delayed because of subtle presentations that do not always present with the classically described clinical findings of this condition or the complexity of the patient with critical illness or injury. Nevertheless, tension pneumothorax should always be a consideration when acute compromise occurs.
Symptoms of tension pneumothorax may include chest pain (90%), dyspnea (80%), anxiety, fatigue, or acute epigastric pain (a rare finding).
Catamenial pneumothorax
Women aged 30-40 years who present with onset of symptoms within 48 hours of menstruation, right-side pneumothorax, and recurrence raise suspicion for catamenial pneumothorax.
Pneumomediastinum
Pneumomediastinum must be differentiated from spontaneous pneumothorax. Patients may or may not have symptoms, as this is typically a well-tolerated disease, although mortality in cases of esophageal rupture is very high.
This condition usually occurs when intrathoracic pressures become elevated, such as with an exacerbation of asthma, coughing, vomiting, childbirth, seizures, and a Valsalva maneuver. In many patients who present with pneumomediastinum, it occurs as a result of endoscopy and small esophageal perforation.
In a retrospective review of cases presenting to an academic medical center, 67% of identified patients had chest pain; 42% had persistent cough; 25% had sore throat; and 8% had dysphagia, shortness of breath, or nausea/vomiting.
Other symptoms may include substernal chest pain, usually radiating to the neck, back, or shoulders and exacerbated by deep inspiration, coughing, or supine positioning; dyspnea; neck or jaw pain; dysphagia, dysphonia, and/or abdominal pain (unusual symptoms).
Traumatic mediastinum, though present in as many as 6% of patients, does not portend serious injury.
Physical Examination
The presentation of a patient with pneumothorax may range from completely asymptomatic to life-threatening respiratory distress. Symptoms may include diaphoresis, splinting chest wall to relieve pleuritic pain, and cyanosis (in the case of tension pneumothorax). Findings on lung auscultation also vary, depending on the extent of the pneumothorax. Affected patients may also reveal altered mental status changes, including decreased alertness and/or consciousness (a rare finding).
Respiratory findings may include the following:
- Respiratory distress (considered a universal finding) or respiratory arrest
- Tachypnea (or bradypnea as a preterminal event)
- Asymmetric lung expansion – A mediastinal and tracheal shift to the contralateral side can occur with a large tension pneumothorax
- Distant or absent breath sounds – Unilaterally decreased or absent lung sounds is a common finding, but decreased air entry may be absent even in an advanced state of the disease
- Lung sounds transmitted from the unaffected hemithorax are minimal with auscultation at the midaxillary line
- Hyperresonance on percussion – This is a rare finding and may be absent even in an advanced state of the disease
- Decreased tactile fremitus
- Adventitious lung sounds (crackles, wheeze; an ipsilateral finding)
Cardiovascular findings may include the following:
- Tachycardia – This is the most common finding; if the heart rate is faster than 135 beats/min, tension pneumothorax is likely
- Pulsus paradoxus
- Hypotension – This should be considered as an inconsistently present finding; although hypotension is typically considered a key sign of a tension pneumothorax, studies suggest that hypotension can be delayed until its appearance immediately precedes cardiovascular collapse
- Jugular venous distention – This is generally seen in tension pneumothorax, though it may be absent if hypotension is severe
- Cardiac apical displacement – This is a rare finding
Spontaneous and iatrogenic pneumothorax
Signs of spontaneous and iatrogenic pneumothorax are similar and depend on the underlying lung disease and extent of the pneumothorax. Tachycardia is the most common finding, and tachypnea and hypoxia may be present.
Tension pneumothorax
Although tension pneumothorax may be a difficult diagnosis to make and may present with considerable variability in signs, respiratory distress and chest pain are generally accepted as being universally present, and tachycardia and ipsilateral air entry on auscultation are also common findings. Sometimes, reliance on history alone may be warranted.
Findings may be affected by the volume status of the patient. In hypovolemic trauma patients with ongoing hemorrhage, the physical findings may lag behind the presentation of shock and cardiopulmonary collapse. Increased pulmonary artery pressures and decreased cardiac output or cardiac index are evidence of tension pneumothorax in patients with Swan-Ganz catheters.
When a patient is being examined for suspected tension pneumothorax, any clue may be helpful; subtle thoracic size and thoracic mobility differences may be elicited by performing careful visual inspection along the line of the thorax. When a patient is supine, the examiner should lower himself or herself to be on a level with the patient.
Tracheal deviation is an inconsistent finding. Although historic emphasis has been placed on tracheal deviation in the setting of tension pneumothorax, tracheal deviation is a relatively late finding caused by midline shift.
Abdominal distention may occur from increased pressure in the thoracic cavity producing caudal deviation of the diaphragm and from secondary pneumoperitoneum produced as air dissects across the diaphragm through the pores of Kohn.
If patients who are mechanically ventilated are difficult to ventilate during resuscitation, high peak airway pressures are clues to pneumothorax. A tension pneumothorax causes progressive difficulty with ventilation as the normal lung is compressed. On volume-control ventilation, this is indicated by marked increase in both peak and plateau pressures, with relatively preserved peak and plateau pressure difference. On pressure-control ventilation, tension pneumothorax causes sudden drop in tidal volume. However, these observations are neither sensitive nor specific for making the diagnosis of pneumothorax or ruling out the possibility of pneumothorax.
The development of tension pneumothorax in patients who are ventilated will generally be of faster onset with immediate and progressive declines in arterial and mixed venous oxyhemoglobin saturation and an immediate decline in cardiac output. Cardiac arrest associated with asystole or pulseless electrical activity (PEA) may ultimately result. Occasionally, the tension pneumothorax may be tolerated and its diagnosis delayed for hours to days after the initial insult. The diagnosis may become evident only if the patient is receiving positive-pressure ventilation (PPV). Tension pneumothorax has been reported during surgery with both single- and double-lumen tubes.
Pneumomediastinum
As with pneumothorax, physical findings of pneumomediastinum may be variable, including absent signs in some patients. However, subcutaneous emphysema is the most consistent sign. Another sign, the Hamman sign—a precordial crunching noise synchronous with the heartbeat and often accentuated during expiration—has a variable rate of occurrence, with one series reporting 10%.
Diagnostic Considerations
This section reviews some important points to consider in the diagnosis of pneumothoraces.
Spontaneous pneumothorax
Because patients with primary spontaneous pneumothorax (PSP) will have apical emphysematous pulmonary disease on computed tomography (CT) or thoracoscopy, they can be thought to have a congenital syndrome of mild acinar emphysema, whose expression is enhanced by environmental factors (eg, smoking), just as it is in patients with alpha-1-antitrypsin deficiency and “typical” emphysema.
Folliculin gene disorders have been described in familial spontaneous pneumothorax. These patients may have pneumothorax as the presenting symptom of Birt-Hogg-Dube disease. Some authors recommend screening patients with a family history of pneumothorax for the benign skin tumors and renal cancers that arise from the disease.
Catamenial pneumothorax is a rare cause of recurrent pneumothorax in women. Prior to recurrence, this condition may initially be diagnosed as PSP.
Pneumonia is a possible cause of pneumothorax; in the patient with human immunodeficiency virus infection (HIV), Pneumocystis jiroveci pneumonia (PCP) ,toxoplasmosis, and Kaposi sarcoma must be considered . A patient with HIV can have spontaneous pneumothorax as the presenting symptom of the illness: HIV carries a lifetime risk of 6% for pneumothorax, and about 85% of that number is related to PCP pneumonia.
The rare event of spontaneous pneumothorax leading to tension pneumothorax may be misdiagnosed as an asthma crisis or exacerbation of chronic obstructive pulmonary disease (COPD) in the patient presenting with tachycardia, subcutaneous emphysema, dyspnea, and shock.
Traumatic pneumothorax
Pneumothorax must always be considered in the differential diagnosis of major trauma. In the patient with blunt trauma and mental status changes, hypoxia, and acidosis, symptoms of a tension pneumothorax may be masked by associated and similarly potentially lethal injuries.
In assessing the trauma patient, it is important to be aware that the clinical presentations of tension pneumothorax and cardiac tamponade may be similar.
Tension pneumothorax
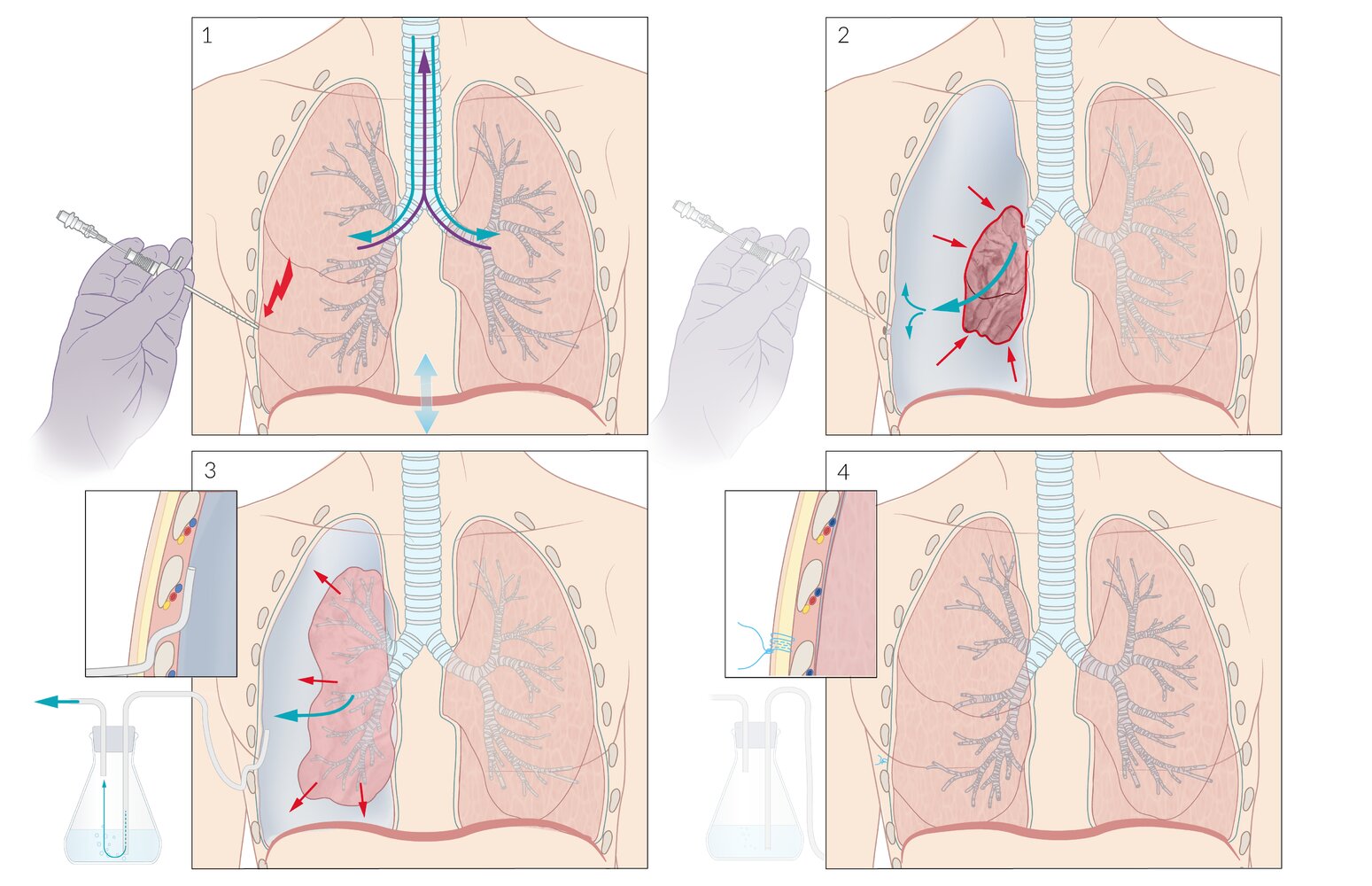
The diagnosis of a tension pneumothorax should largely be based on the history and physical examination findings. Ultrasonography (US) in the emergency setting is increasingly being used as an adjunct to the physical examination when there is doubt regarding the diagnosis. Chest radiography or CT should be used only in those instances when the diagnosis is in doubt and when the patient is hemodynamically stable. Obtaining such imaging studies when the diagnosis of tension pneumothorax is not in question causes an unnecessary and potentially lethal delay in treatment.
A tension pneumothorax is a life-threatening condition and warrants immediate action (eg, needle thoracostomy or chest tube insertion). However, the clinician should be wary of prematurely diagnosing a tension pneumothorax in a patient without respiratory distress, hypoxia, hypotension, or cardiopulmonary compromise. If the clinical presentation is questionable and the patient appears stable, the clinician should reexamine the patient and use bedside US or request immediate portable chest radiography (or reexamine the chest radiographs if they have already been obtained) to confirm the diagnosis.
A high index of suspicion for tension pneumothorax is recommended in patients on mechanical ventilation with acute onset of hemodynamic instability, difficult ventilation with high inspiratory pressures, and worsening hypoxemia and/or hypercapnia, even with a functioning chest tube in place. Patients at greatest risk for pneumothorax and/or tension pneumothorax include the following:
- Those with COPD who are using ventilators
- Those with acute respiratory distress syndrome (ARDS)
- Those receiving a tidal volume greater than 12 mL/kg, a peak airway pressure greater than 60 cm H 2O, or a positive end-expiratory pressure greater than 15 cm H 2O
Portable chest radiography may fail to show the pneumothorax; CT may be required for diagnosis.
Avoid assuming that a patient with a chest tube does not have a tension pneumothorax if he or she has respiratory or hemodynamic instability. Chest tubes can become plugged or malpositioned and cease to function. In addition, improper attachment of a one-way valve to the chest tube may produce tension pneumothorax.
Additional considerations
Other conditions to consider include the following:
- Aspiration, bacterial, mycoplasmal, and viral pneumonia
- Asthma
- Costochondritis
- Diaphragmatic injuries
- Esophageal spasm
- Foreign bodies, trachea
- Mediastinitis
- Myocardial ischemia
- Myocarditis
- Pericarditis
- Pleurodynia
- Pulmonary empyema and abscess
- Tuberculosis
Differential Diagnoses
Acute Aortic Dissection
Acute Coronary Syndrome
Acute Pericarditis
Esophageal Rupture and Tears in Emergency Medicine
Heart Failure
Myocardial Infarction
Pediatric Acute Respiratory Distress Syndrome
Pulmonary Embolism (PE)
Rib Fracture
Approach Considerations
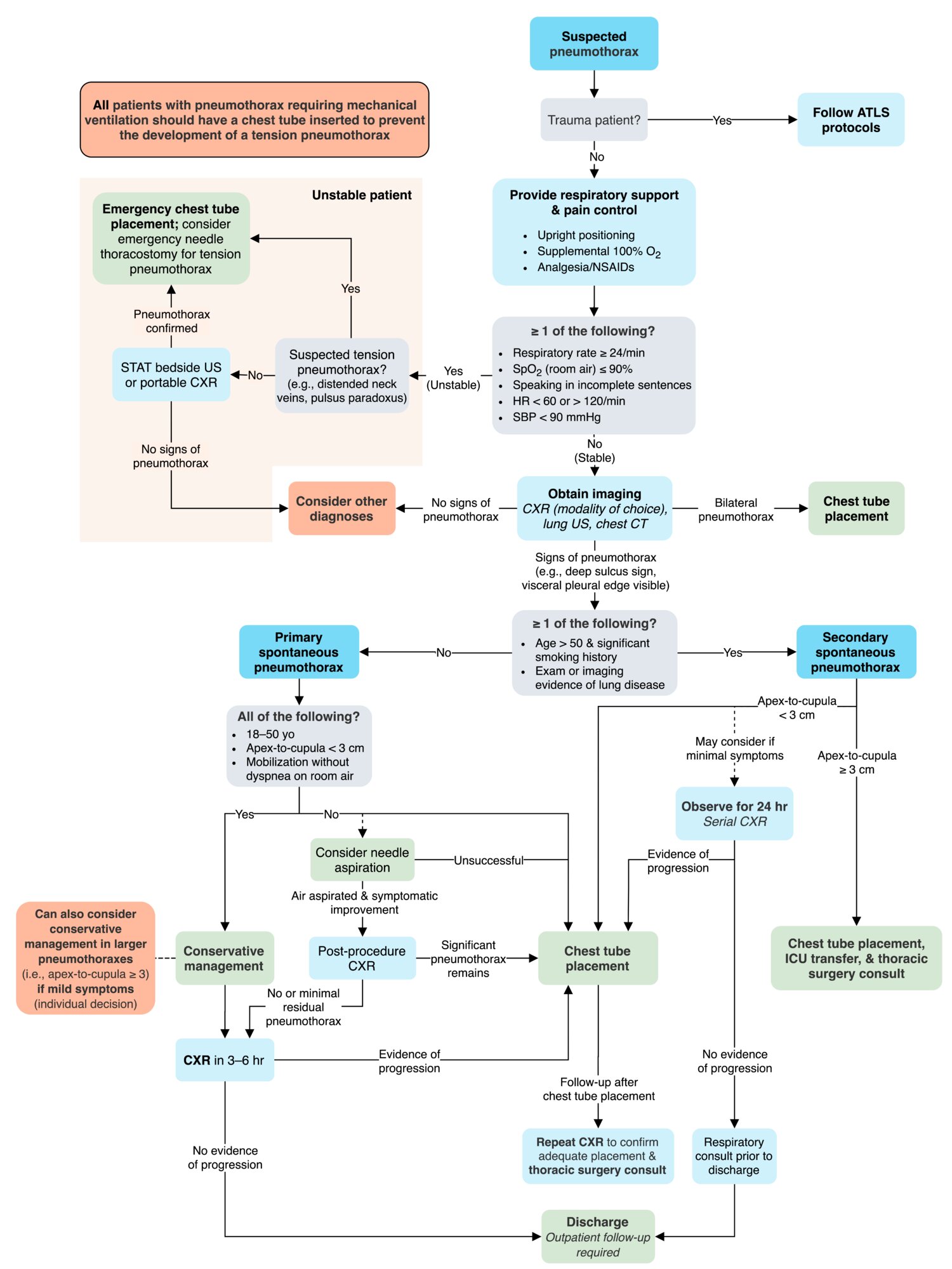
History and physical examination remain the keys to making the diagnosis. When pneumothorax is suspected, confirmation by means of chest radiography affords additional information beyond confirmation, such as the extent of pneumothorax, potential causes, a baseline study from which to go forward, and assistance with the therapeutic plan.
In the evaluation of trauma patients, airway and breathing collectively are the primary concern. Portable chest radiography is virtually always included in the initial radiographic evaluation of a major trauma victim, because significant chest injuries may be masked by lack of physical findings or associated injuries. Chest trauma carries an estimated 10-50% risk of associated pneumothorax.
Computed tomography (CT) of the chest likewise should always be performed for significant chest injuries, because plain radiography may miss associated thoracic trauma. Specifically for pneumothorax, the presence of a pneumothorax seen only on CT defines it as occult. In stable patients, however, chest radiography is often unnecessary.
Tension pneumothorax is a clinical diagnosis that now is more readily recognized because of improvements in emergency medical services (EMS) and the widespread application of educational programs such as Advanced Trauma Life Support (ATLS) and Fundamental Critical Care Support (FCCS).
Although laboratory and imaging studies help determine a diagnosis, as discussed earlier, tension pneumothorax primarily is a clinical diagnosis based on patient presentation. Suspicion of tension pneumothorax, especially in late stages, mandates immediate treatment and does not require potentially prolonged diagnostic studies (see the image below).

Chest radiograph shows two abnormalities: (1) tension pneumothorax and (2) potentially life-saving intervention delayed during wait for x-ray results. Tension pneumothorax is clinical diagnosis requiring emergency needle decompression, and therapy should never be delayed for x-ray confirmation.
Arterial Blood Gas Analysis
Arterial blood gas (ABG) studies measure the degrees of acidemia, hypercarbia, and hypoxemia, the occurrence of which depends on the extent of cardiopulmonary compromise at the time of collection.
In patients with severe underlying lung disease and in those with persistent respiratory distress despite treatment, hypoxemia not only occurs with an increased alveolar-arterial oxygen tension gradient but also tends to be more severe in patients with secondary spontaneous pneumothoraces.
ABG analysis does not replace physical diagnosis, nor should treatment be delayed to await results if symptomatic pneumothorax is suspected. However, ABG analysis may be useful in evaluating hypoxia and hypercarbia and respiratory acidosis.
Chest Radiography
When evaluating the chest radiograph for pneumothorax, one should use a systematic approach. Rotation, which can obscure a pneumothorax and mimic a mediastinal shift, should always be assessed. The clavicles should be compared with respect to symmetry and shape, and the relative lengths of the ribs in the middle lung fields on each side on the anteroposterior (AP) or posteroanterior (PA) views should be assessed. On an image with rotation, the ribs on each side often have unequal lengths.
In a nonloculated pneumothorax, air generally rises to the nondependent portion of the pleural cavity. Therefore, carefully examine the apices on an upright chest radiograph should be carefully examined, and the costophrenic and cardiophrenic angles on a supine chest radiograph should be scrutinized.
Finding of pneumothorax on chest radiographs may include the following:
- A linear shadow of visceral pleura with lack of lung markings peripheral to the shadow may be observed, indicating collapsed lung
- An ipsilateral lung edge may be seen parallel to the chest wall
- In supine patients, a deep sulcus sign (very dark and deep costophrenic angle) with radiolucency along costophrenic sulcus may help identify an occult pneumothorax; the anterior costophrenic recess becomes the highest point in the hemithorax, resulting in an unusually sharp definition of the anterior diaphragmatic surface due to gas collection and a depressed costophrenic angle
- Small pleural effusions commonly are present and increase in size if the pneumothorax does not reexpand
- Mediastinal shift toward the contralateral lung may also be apparent
- Airway or parenchymal abnormalities in the contralateral lung suggest causes of secondary pneumothorax; evaluation of the parenchyma in the collapsed lung is less reliable
Although expiratory images are thought to be better for depicting subtle pneumothoraces (the volume of the pneumothorax is constant and hence proportionally higher on expiratory images), a randomized controlled trial revealed no difference in the ability of radiologists to detect pneumothoraces on inspiratory and expiratory images after procedures with the potential to cause pneumothoraces. (See the images below.)
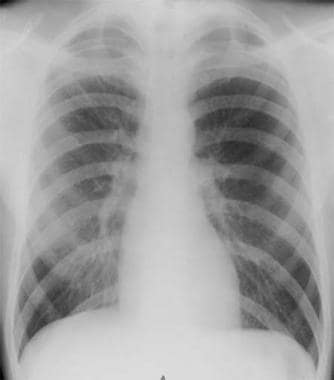
Radiograph of patient with small spontaneous primary pneumothorax.
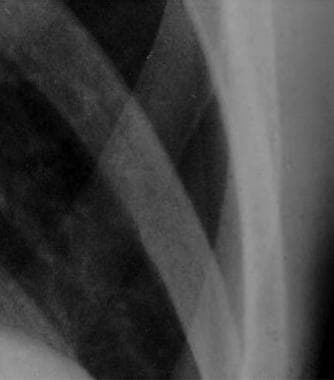
Close radiographic view of patient with small spontaneous primary pneumothorax (same patient as in previous image).
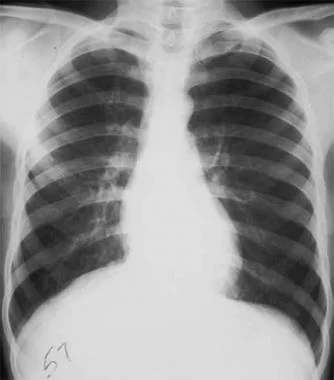
Radiograph of patient with spontaneous primary pneumothorax due to left-upper-lobe bleb.
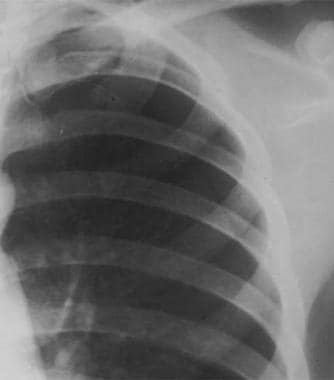
Close radiographic view of patient with spontaneous primary pneumothorax due to left-upper-lobe bleb (same patient as in previous image).
Tension pneumothorax
Imaging studies should not delay the diagnosis and treatment of tension pneumothorax; this condition is a medical emergency. When radiography is being considered, the use of a risk-benefit analysis has been suggested, in which the time taken to obtain the radiograph is balanced against the expected clinical course, with decompression preceding chest radiography in ventilated patients who are prone to rapid decompensation.
In a very select subset of patients, it may be preferable to confirm and localize tension pneumothorax radiologically before subjecting the patient to potential morbidities arising from decompression. The relevant subset of patients consists of those who are awake, stable, and not in any distress and in whom an immediate chest film can be obtained, with a continuously accompanying clinician ready to perform urgent decompression should the need arise.
In the rare case that a chest radiograph is obtained safely, findings can include ipsilateral lung collapse at the hilum, increased thoracic volume, trachea and mediastinum deviation to the contralateral side, widened intercostal spaces on the affected side, heart border ipsilateral flattening. With a left hemithorax, the left hemidiaphragm may be depressed, but the liver prevents this occurrence on the right side. (See the images below.)
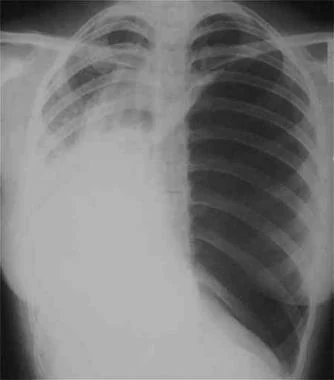
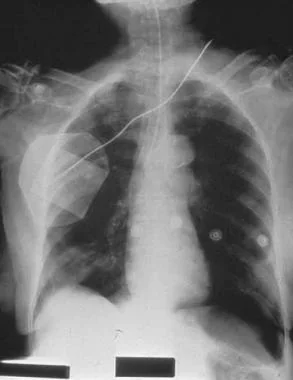
Radiograph of patient with large spontaneous tension pneumothorax.
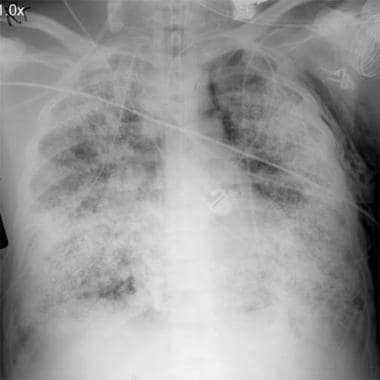
Radiograph of new left-side pneumothorax in patient on mechanical ventilation, requiring high inflation pressures.

Pneumomediastinum from barotrauma may result in tension pneumothorax and obstructive shock.
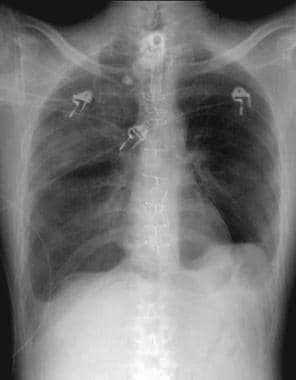
Radiograph of patient in ICU who developed pneumopericardium as manifestation of barotrauma.
Radiograph showing right mainstem intubation that resulted in left-side tension pneumothorax, right mediastinal shift, deep sulcus sign, and subpulmonic pneumothorax.
Although the initial chest radiograph may show no evidence of pneumothorax, it is essential to consider the possibility of delayed traumatic pneumothorax developing in any penetrating chest wound. Stable patients without pneumothorax on initial films can be observed with serial chest radiographs at 3 hours after injury to rule this out.
Pneumomediastinum
Mediastinal emphysema appears as a thin line of radiolucency that outlines the cardiac silhouette, as well as thin, lucent, vertically oriented streaks of air within the mediastinum (see the image below). The aorta and other posterior mediastinal structures are highlighted, and a well-defined lucency around the right pulmonary artery (“ring around the artery” sign) may be seen.
Air most easily is detected retrosternally on the lateral chest radiograph. An AP chest radiograph may not depict the finding in 50% of cases. An expiratory radiograph may help detect small apical pneumothoraces. Unlike the air in pneumothorax or pneumopericardium, the air in pneumomediastinum remains fixed and does not rise to the highest point.
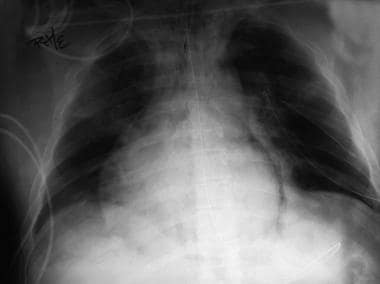
Chest radiograph shows pneumomediastinum (radiolucency noted around left heart border) in patient who had respiratory and circulatory arrest in emergency department after experiencing multiple episodes of vomiting and rigid abdomen. Patient was taken immediately to operating room, where large rupture of esophagus was repaired.
Estimating the size of the pneumothorax
In evaluating the chest radiograph, first impressions of pneumothorax size can be misleading. The following methods may be used to estimate the size of the pneumothorax:
- Calculate the ratio of the transverse radius of the pneumothorax (cubed) to the transverse radius of the hemithorax (cubed); to express the pneumothorax size as a percentage, multiply the fractional size by 100 (this formula assumes a constant shape of the lung when it collapses and is invalid if pleural adhesions are present); the ratio of lung size to hemithorax size to estimate pneumothorax size avoids the subjective underestimation of pneumothorax expressed as a percentage of previous lung volume
- A 2.5-cm margin of gas peripheral to the collapsing lung corresponds to a pneumothorax of about 30%; complete collapse of the lung is a 100% pneumothorax
- A simple approach involves measuring the distance from the apex of the lung to the top margin of the visceral pleura (thoracic cupola) on the upright chest radiograph, so that a small pneumothorax is a distance to the apex that measures less than 3 cm and large pneumothorax has greater than 3 cm distance to the apex
The cut point distinguishing small and large pneumothoraces has varied somewhat among professional societies and experts. The British Thoracic Society (BTS) has used 2 cm as the cutoff, the American College of Chest Physicians (ACCP) has used 3 cm as the cut point, and the Light Index has used 15% of the thoracic volume on the posterior-anterior film as the cut point.
Disadvantages of chest radiographs
Chest radiographs may fail to reveal pneumothorax or radiologists or interpreting physicians may fail to recognize the presence of the pneumothorax. Other disadvantages are as follows:
- In patients with underlying pulmonary disease, the classic visceral pleural line may be harder to detect, because the lung is hyperlucent, and little difference exists in the radiographic density between the pneumothorax and the emphysematous lung
- A vertical skin line can be mistaken for a pneumothorax, leading to unnecessary and possibly harmful therapy
- Large bulla can simulate pneumothorax on chest radiographs, so that CT may be required to clarify the diagnosis
- Occasionally, skin folds, the scapula, and bed sheets can mimic the pleural line, falsely suggesting pneumothorax on the chest radiograph; unlike pneumothoraces, skin folds usually continue beyond the chest wall, and lung markings can be seen peripheral to the skin fold line; viewing the film under the hot lamp may be necessary to discern obscure peripheral lung markings
As ultrasonography (US) becomes increasingly available in emergency situations, the already limited role of radiography in tension pneumothorax will be further minimized. Multiple studies have shown bedside US to be more accurate than supine chest radiography in detecting and quantifying the presence of pneumothorax, including traumatic pneumothorax.
Other Radiographs and Transillumination
Confirmation of a suspected pneumothorax that is not readily observed on standard supine anteroposterior (AP) radiograph can be demonstrated by obtaining a lateral decubitus film with the involved hemithorax positioned uppermost (see the images below).
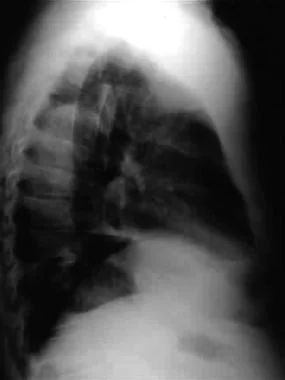
Lateral radiograph demonstrating tension and traumatic pneumothorax.
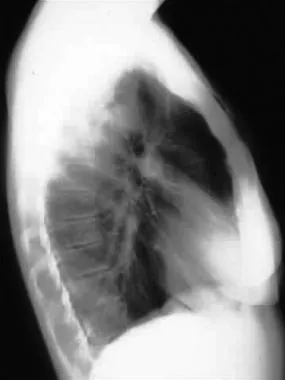
Lateral radiograph demonstrating tension and traumatic pneumothorax.
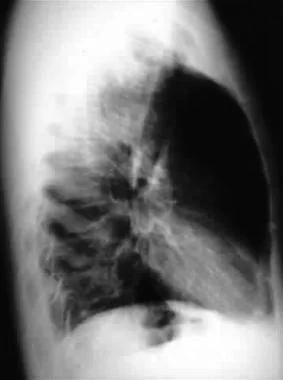
Lateral radiograph showing tension and traumatic pneumothorax.
In neonatal patients, transillumination may reveal an increased transmission of light through the chest on the affected side.
Contrast-Enhanced Esophagography
If emesis or retching is the precipitating event for a pneumothorax, an esophagogram should be obtained to evaluate for Boerhaave syndrome (an esophageal tear), which has a high mortality. This is the study of choice in all cases of suspected esophageal perforation (ie, postendoscopy patients). Esophagoscopy could further be performed for esophageal perforations.
Computed Tomography of Chest
Chest CT is the most reliable imaging study for the diagnosis of pneumothorax, but it is not recommended for routine use in pneumothorax. This imaging modality can help to accomplish the following:
- Distinguish between a large bulla and a pneumothorax
- Indicate underlying emphysema or emphysemalike changes (ELCs)
- Determine the exact size of the pneumothorax, especially if it is small
- Confirm the diagnosis of pneumothorax in patients with head trauma who are mechanically ventilated
- Detect occult or small pneumothoraces and pneumomediastinum (though the clinical significance of these occult pneumothoraces is unclear, particularly in the stable nonintubated patient)
CT is widely used in actual clinical practice to assess the possibility of associated concurrent pulmonary disease because of the inherent superiority of CT for visualizing the details of lung parenchyma and pleura (see the images below).
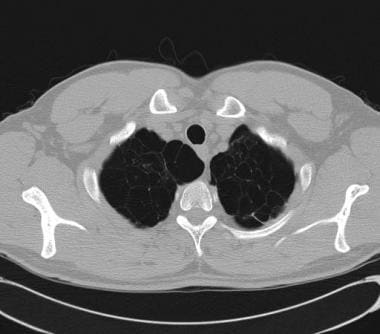
CT scan demonstrating blebs in patient with chronic obstructive pulmonary disease (COPD).
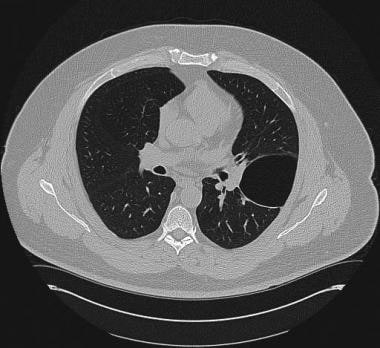
CT scan demonstrating bulla in asymptomatic patient.
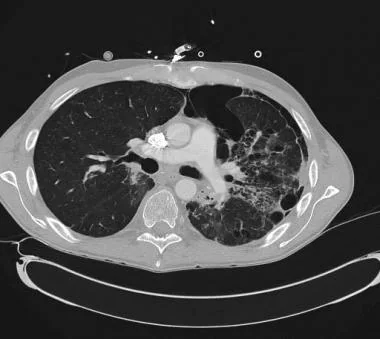
CT scan demonstrating secondary spontaneous pneumothorax (SSP) from radiation/chemotherapy for lymphoma.
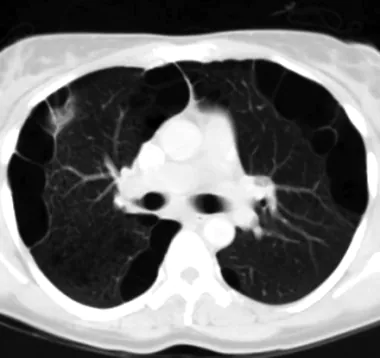
CT scan demonstrating emphysematouslike changes (ELCs) in patient with chronic obstructive pulmonary disease (COPD).
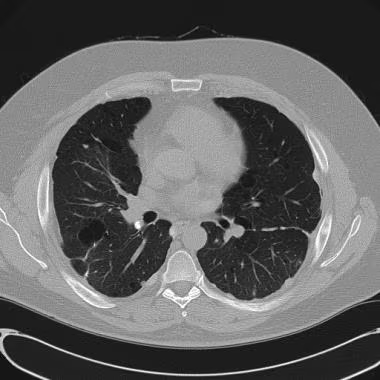
CT scan in patient with history of bilateral pleurodesis and strong family history of spontaneous pneumothorax.
Spontaneous pneumothorax
When performed on primary spontaneous pneumothorax patients, CT detects multiple blebs and bullae in the setting of negative chest radiographic findings. This may not impact management, in that there has been no correlation between number of blebs and recurrence. However, CT may have a role in secondary spontaneous pneumothorax (SSP), especially for differentiating SSP from giant bullous emphysema.
Traumatic and tension pneumothorax
CT can detect occult pneumothorax in patients in trauma and emergency surgery settings. If the patient requires mechanical ventilation and/or anesthesia, all participants should be made aware of the findings; sometimes, prophylactic tube thoracostomy may be performed. This modality has also been shown to be more sensitive than radiography for hemothorax and pulmonary contusion.
Collapse of the lung, air in the pleural cavity, and deviation of mediastinal structures are present in tension pneumothorax.
Pneumomediastinum
CT may improve diagnostic sensitivity in pneumomediastinum and should be obtained if clinical suspicion is present for this condition. One small study suggested that mild pneumomediastinum was underdiagnosed based on chest radiographic findings, and CT was needed to make the diagnosis.
Ultrasonography
Prehospital portable US (US) may provide diagnostic and therapeutic benefit when conducted by a proficient examiner who uses goal-directed and time-sensitive protocols, as determined in an air rescue setting. Further study in this area may help to determine the indications and role of prehospital US. In experienced hands, US may be quicker and more accurate than radiography for distinguishing free pleural effusion (a finding in pneumothorax) in time-sensitive evaluations.
US is increasingly employed in the acute care setting as a readily available bedside tool, especially in the intensive care unit (ICU) and the emergency department (ED). It provides a rapid imaging option for diagnosis of pneumothorax, but this evaluation should not delay treatment of a clinically apparent tension pneumothorax.
Many trauma centers incorporate chest US as an adjunct to the Focused Assessment with Sonography in Trauma (FAST) examination. Knudtson et al, in a prospective analysis of 328 consecutive trauma patients at a level 1 trauma center, obtained a specificity of 99.7% and an accuracy of 99.4%, and concluded that US was a reliable modality for the diagnosis of pneumothorax in the injured patient.
A prospective study by Brook et al designed to assess the accuracy of radiology residents in detecting pneumothoraces as part of the extended FAST (eFAST) examination concluded that ultrasonographic pneumothorax detection by these radiology residents was both accurate and efficient in the early detection of clinically important pneumothoraces.
The investigators compared ultrasonographic pneumothorax detection (by the absence of parietal-over-visceral lung sliding with “comet tail” artifacts behind it) with the reference standard of chest CT in 169 consecutive trauma patients (ie, 338 lung fields). A sensitivity of 47%, specificity of 99%, positive predictive value of 87%, and negative predictive value of 93% was found; none of the small pneumothoraces missed by US required drainage during the hospitalization period.
In addition, Hernandez et al noted that US is the only radiographic modality allowing patients with nonarrhythmogenic cardiac arrest to continue undergoing resuscitation while clinicians search for easily reversible causes of asystole or pulseless electrical activity (PEA). They proposed further investigation into the CAUSE (Cardiac Arrest UltraSound Exam) protocol, in which cardiac arrest patients, concurrent with resuscitation, receive bedside US to look for cardiac tamponade, massive pulmonary embolus, severe hypovolemia, and tension pneumothorax. The adoption of US in this setting may enhance “real-time” diagnostic acumen and shorten the time to appropriate condition-related therapy.
Notable features
Features of US examination for the diagnosis of pneumothorax include absence of lung sliding (high sensitivity and specificity), absence of comet-tail artifact (high sensitivity, lower specificity), and presence of lung point (high specificity, lower sensitivity).
In the absence of pleural disease, visceral pleura moves against parietal pleura while breathing. This movement of the two pleurae is detected by US as lung sliding, which is a “kind of twinkling synchronized with respiration” seen in real-time and time-motion modes. That is, lung sliding refers to normal pleural movement in patients without pneumothorax. One study showed that absent lung sliding from an anterior approach indicated pneumothorax with 81% sensitivity and 100% specificity.
Comet-tail artifacts are vertical air artifacts that arise from the visceral pleural line (or, in the case of parietal emphysema or shotgun pellets, may arise above the pleural line). Lung point is the location that lung-sliding and absent lung-sliding alternately appear; it has been shown in multiple studies to allow determination of the size of a pneumothorax. Zhang et al obtained a 79% sensitivity in lung point’s ability to determine pneumothorax size.
Advantages
US has high sensitivity (95.65%), specificity (100%), and diagnostic effectiveness (98.91%) for pneumothorax when CT is used as the criterion standard. In another study, US performed on patients with blunt thoracic trauma had 94% sensitivity and 100% specificity for pneumothorax detection compared with spiral CT.
This imaging modality can be used as a possible bedside technique to detect pneumothorax, which may be useful in unstable patients. A prospective study involving 135 patients with multiple trauma using bedside US performed by ED clinicians obtained 86% sensitivity and 97% specificity for the detection of pneumothorax. Traumatic pneumothorax in the ICU setting can also be followed accurately and early (initial 24 hr) with US alone for resolution of the lesion. US does not use ionizing radiation and is repeatable.
Disadvantages
US is heavily operator-dependent. In addition, this modality cannot be used to discriminate between a chronic obstructive pulmonary disease (COPD)-associated bleb and pneumothorax.
The sensitivity of US drops in the ICU, especially in patients with acute respiratory distress syndrome (ARDS), Moreover, in a preliminary study by Dente et al, though US evaluation for pneumothorax was found to be very accurate for the first 24 hours after insertion of a thoracostomy tube, its accuracy was not sustained over time. By 24 hours after thoracostomy, the diagnostic sensitivity of US for pneumothorax had fallen to 55%, and its positive predictive value to 43%. This may be related to intrapleural adhesion formation.
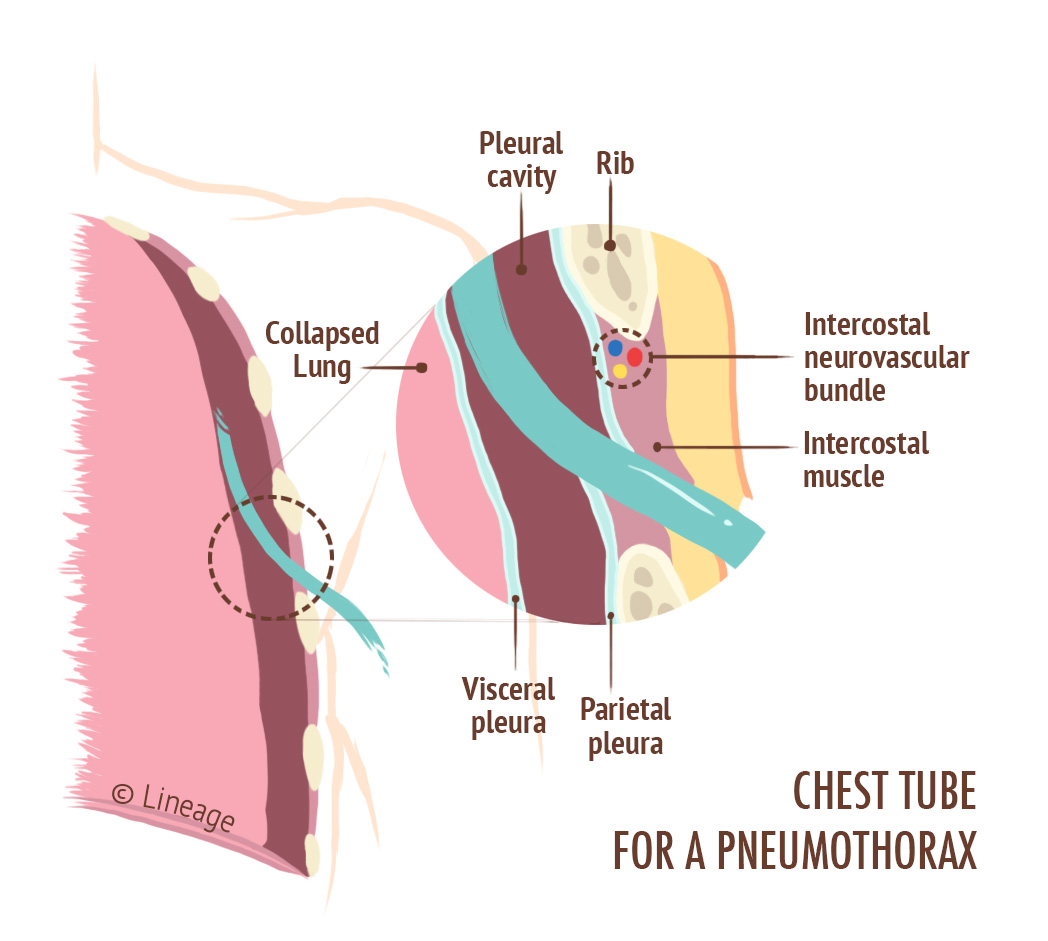
Treatments and Managements
Despite general agreement on the management of pneumothorax, a full consensus about management of initial or recurrent pneumothorax does not exist. Professional societies differ in their approach to management and hospitalization.
This section presents a risk stratification framework as well as other approaches for choosing among options to restore lung volume and an air-free pleural space and to prevent recurrences. These goals are applicable across diverse clinical presentations. The range of therapeutic options includes watchful waiting with or without supplemental oxygen, simple aspiration, tube drainage with or without medical pleurodesis, video-assisted thoracoscopic surgery (VATS) with pleurodesis and/or closure of leaks and bullectomy, and open surgical procedures such as thoracotomy for pleurectomy or pleurodesis.
Selection among the various management options requires an understanding of the natural history of pneumothorax, the risk of recurrent pneumothorax, and the benefits and limitations of each treatment option and discussion with the patient.
Pharmacotherapy
Medication may be necessary to treat a pulmonary disorder that causes the pneumothorax. For example, intravenous (IV) antibiotics are included in the treatment of a pneumothorax that developed as a sequela of staphylococcal pneumonia. In addition, studies suggest that the administration of prophylactic antibiotics during chest tube insertion may reduce the incidence of complications such as emphysema. Clearly, the use of analgesics can provide patient comfort until the thoracostomy tube is removed. Some authors advocate the use of intercostal nerve blocks to increase patient comfort and decrease the need for narcotic analgesics.
In patients with repeated pneumothoraces who are not good candidates for surgery, sclerotherapy with talc or doxycycline may be necessary.
Treatment Based on Risk Stratification
The decision to observe or to treat with an immediate intervention should be guided by a risk stratification that considers the patient’s presentation and the likelihood of spontaneous resolution and recurrence.
Patient presentation
The following are possible presentations of patients with pneumothorax:
- Asymptomatic (incidental finding) – Treatment decisions are guided by estimate of long-term recurrence risk.
- Symptomatic but clinically stable – Treatment is guided by local resources and conventions for the site of care; the British Thoracic Society (BTS) advocated for conservative management of primary spontaneous pneumothorax (PSP) as initial treatment if the patient is stable ; a small-bore catheter or chest tube placement was recommended by the American College of Chest Physicians (ACCP) Delphi consensus statement
- Clinically fragile – Treatment is guided by local practice patterns for air evacuation and observation; comorbid conditions may preclude observation because of decreased cardiopulmonary reserve
- Life-threatening – Pneumothorax that causes hemodynamic instability is life-threatening and must be treated immediately with tube thoracostomy; all documents and recommendations call for intervention if a patient is unstable
Likelihood of resolution
The likelihood of a pneumothorax resolving is classified as follows:
- Very likely to resolve – Small pneumothorax in a hemodynamically stable patient without significant parenchymal lung disease; small iatrogenic pneumothorax
- May resolve – Large pneumothorax in a normal lung (eg, PSP or iatrogenic pneumothorax)
- Unlikely to resolve – Secondary pneumothorax, enlarging pneumothorax (suggests a continuing air leak)
- Will not resolve, could be fatal – Tension pneumothorax; unrecognized air leak
Likelihood of recurrence
The following categories of pneumothorax recurrence likelihood should be assessed:
- Unlikely to recur – Iatrogenic pneumothorax in normal lung
- May recur, but patient will likely be clinically stable
- May recur and the patient may be clinically unstable, but emergency care is readily accessible
- Very likely to recur – Diffuse and progressive pulmonary pathology (eg, lymphangioleiomyomatosis [LAM])
- Recurrence could be life-threatening – Poor cardiopulmonary reserve, limited access to emergency medical care
Selection of site of patient care
The following is a suggested guideline on determining where to administer care in a patient with a pneumothorax:
- Outpatient care – This can occur in asymptomatic patients or those with a small pneumothorax and reliable follow-up
- Emergency department (ED) care – Prolonged periods of observation are inefficient and clinically suboptimal; efficacy studies of manual aspiration and placement of one-way valves performed in EDs are an attempt to address these practical issues
- Inpatient care – This site of care is generally selected when high-flow oxygen is needed, the pneumothorax is larger but the patient is stable, or comorbidities increase concern about risk or follow-up; the average hospital stay is 2.8 days
- Intensive care unit (ICU) – ICU treatment and observation is appropriate for patients who are unstable or intubated
Interval of observation
No protocols regarding serial radiography or imaging exist; the clinician typically reviews serial vital signs and clinical assessments, using the direction and rate of change in the patient’s clinical status to select imaging frequency. Monitoring pneumothorax size during this time is important, as follows:
- At 0-6 hours – The ACCP Delphi consensus statement recommended observation in an ED for 6 hours, and discharge to home if a follow-up chest radiograph shows no enlargement of the lesion, in reliable patients ; ED observation with a repeat radiograph 6 hours later used to be common but may be used less often now
- At 24-96 hours – Additional follow up in 2 days is recommended, with preference given to a 24-48 hour follow-up radiograph in the outpatient setting; outpatient follow-up during the 96-hour window is essential to distinguish between a resolved pneumothorax and one that needs evacuation; computed tomography (CT) at this time distinguishes between PSP and secondary spontaneous pneumothorax (SSP)
- At 1 month – Full lung reexpansion can occur, on average, 3 weeks after the initial event
Options for Restoring Air-Free Pleural Space
Several options are available to restore an air-free pleural space, including observation without oxygen, administering supplemental oxygen, simple aspiration, chest tube placement, one-way valve insertion, and thoracostomy with continuous suction.
Contou et al recommended that clinicians consider drainage via a small-bore catheter as a first-line treatment for pneumothorax of any cause. The authors found that drainage via catheter or via chest tube is similarly effective for the management for the management of pneumothoraces, including primary spontaneous pneumothorax, secondary spontaneous pneumothorax, and traumatic and iatrogenic pneumothoraces.
Observation without oxygen
Simple observation is appropriate for asymptomatic patients with a minimal pneumothorax (< 15-20% by Light criteria; 2-3 cm from apex to cupola by alternate criteria) with close follow-up, ensuring no enlargement (see Chest Radiography). Air is reabsorbed spontaneously by 1.25% of pneumothorax size per day.
A multicenter, prospective, observational study reported on more than 500 trauma patients with occult pneumothorax identified on CT with an initially normal chest radiograph. Controversy exists in the literature on the treatment of all patients with occult pneumothorax regarding whether to closely observe patients with occult pneumothorax or whether to place a chest tube. This is even more controversial in patients on positive-pressure ventilation (PPV). It is generally accepted after trauma to treat pneumothorax seen on chest radiographs with tube thoracostomy. Conversion to tension pneumothorax is the worst feared complication if left untreated.
The study arms included observation versus tube thoracostomy. Only 6% of patients failed observation and developed pneumothorax, including only 15% failed observation on PPV. In multivariate regression analysis, failure of observation was seen in patients with chest radiographic evidence of pneumothorax progression and symptoms of respiratory distress. According to this study, it is safe to closely observe trauma patients with occult pneumothorax on chest radiographs, even if they are receiving mechanical ventilation.
Supplemental oxygen
Oxygen administration at 3 L/min nasal canula or higher flow treats possible hypoxemia and is associated with a fourfold increase in the rate of pleural air absorption as compared with room air alone.
Simple aspiration
Simple aspiration in 131 cases of small spontaneous pneumothorax yielded successful results up to 87%. Other studies described more limited success in as many as 70% cases. A subsequent ED study found needle aspiration to be as safe and effective as chest tube placement for PSP, conferring the additional benefits of shorter length of stay and fewer hospital admissions.
Chest tube placement
A tube inserted into the pleural space is connected to a device with one-way flow for air removal. Examples of such devices are Heimlich valves and water seal canisters, as well as tubes connected to wall suction devices.
One-way valve insertion (portable system)
The typical goal of inserting one-way valve systems is to avoid hospital admission and still treat the spontaneous pneumothorax. One-way valves may also expedite hospital discharge and be used during transport of an injured patient.
A Heimlich valve is a one-way rubber flutter valve that allows complete evacuation of air that is not under tension. The proximal end attaches to the chest tube or catheter, and the distal end connects to a suction device or is left open to the atmosphere. Heimlich valves do not require suction and thus eliminate the chance of a tension pneumothorax; they also allow greater mobility and less discomfort for the patient. By decreasing the length of the hospital stay and allowing for outpatient care, medical costs are reduced as well.
In a pilot study, Marquette et al determined that using a serial-steps approach with a single system (small-caliber catheter/Heimlich valve) in patients with a first episode of PSP was as effective as simple manual needle aspiration or a conventional tube thoracostomy. In 41 thin, young, smoking males, a one-way Heimlich valve was connected to the catheter, allowing the air to flow spontaneously outward for 24-48 hours; thereafter, if the lung failed to reexpand, wall suction was applied. Patients with an air leak persisting for more than 4 days were referred for surgery.
At 24 hours, the success rate was 61%, and at 1 week, it had risen to 85%; the actuarial 1-year recurrence rate was 24%. When 24-hour and 1-week success rates and recurrence at 12 months were taken as end points, the method described above was effective as simple manual needle aspiration or a conventional chest tube thoracotomy.
Heimlich valves are widely used in the care of patients with AIDS who have a median length of 20 days of chest tube placement to facilitate care and mobility.
Thoracostomy with continuous wall suction
First-time secondary SSP (including chronic obstructive pulmonary disease [COPD]) and traumatic pneumothorax typically require this approach. A small-bore catheter (eg, 7-14 French) is safe to use in most patients, whereas a larger chest tube (24 French) is also appropriate initially, and increasing suction pressure can be used if the lung fails to inflate.
A larger tube (eg, 28 French) can reduce resistance in patients who are ventilated and at greater risk for air leaks. Air leaks resolve within 7 days of treatment 80% of the time, with an average hospital stay of 5 days. The tube should be kept in place for 24 hours after the air leak ceases.
Prehospital Care
The ABCs (airway, breathing, circulation) should be assessed, and the possibility of a tension pneumothorax should be considered. Vital signs should be evaluated and pulse oximetry performed. A tension pneumothorax is almost always associated with hypotension.
Oxygen should be administered to the patient, ventilation initiated, and IV access established.
Tension pneumothorax
Failure of the emergency medical service personnel (EMS) and medical control physician to make a correct diagnosis of tension pneumothorax and to perform needle decompression promptly in the prehospital setting can result in rapid clinical deterioration and cardiac arrest. Most paramedics are trained and protocolized to perform needle decompression for immediate relief of a tension pneumothorax.
If, however, an incorrect diagnosis of tension pneumothorax is made in the prehospital setting, the patient’s life may be endangered by unnecessary invasive procedures. Close cooperation and accurate communication between the ED and the EMS personnel are of paramount importance.
To prevent reentry of air into the pleural cavity after needle thoracostomy and decompression in the prehospital setting, a one-way valve should be attached to the distal end of the Angiocath. If available, a Heimlich valve may be used. If a commercially prepared valve is not available, attach a finger condom or the finger of a rubber glove with its tip removed to serve as a makeshift one-way valve device.
Clothing covering a wound that communicates with the chest cavity can play a role in producing a one-way valve effect, allowing air to enter the pleural cavity but hindering its exit. Removing such clothing items from the wound may facilitate decompression of a tension pneumothorax.
A tension pneumothorax is a contraindication to the use of military antishock trousers.
Prehospital ultrasonography
In a preliminary 2006 study from Norway, Busch evaluated the feasibility of using portable ultrasound devices in an air rescue setting, concluding that prehospital ultrasonography (US) could provide diagnostic and therapeutic benefit when conducted by a proficient examiner who used goal-directed and time-sensitive protocols. Further study in this area will more fully define the indications for and role of prehospital US.
Hospital Management
Immediate attention to the ABCs in conjunction with assessment of vital signs and oxygen saturation is paramount, particularly in patients with thoracic trauma. The following are essential:
- Evaluate the patency of the airway and the adequacy of the ventilatory effort
- Assess the circulatory status and the integrity of the chest wall
- Carefully evaluate the cardiovascular system, because a tension pneumothorax and pericardial tamponade can cause similar findings
ED care depends on the hemodynamic stability of the patient. All patients should receive supplemental oxygen to increase oxygen saturation and to enhance the reabsorption of free air.
It should be kept in mind that US is the only imaging modality that allows patients with nonarrhythmogenic cardiac arrest to continue undergoing resuscitation while clinicians search for easily reversible causes of asystole or pulseless electrical activity (PEA). Further investigation of the CAUSE (Cardiac Arrest UltraSound Exam) protocol, in which cardiac arrest patients, concurrently with resuscitation, receive bedside US to look for cardiac tamponade, massive pulmonary embolus, severe hypovolemia, and tension pneumothorax, has been proposed. (See Ultrasonography.)
Primary and secondary spontaneous pneumothorax
If the PSP is smaller than 15% (or estimated as small) and the patient is symptomatic but hemodynamically stable, needle aspiration is the treatment of choice.
If the PSP is smaller than 15% and if the patient is asymptomatic, many consider observation to be the treatment of choice. (If the patient is admitted, oxygen should be administered; this has been shown to speed resolution of the pneumothorax.)
If the PSP is greater than 15% (or estimated as large) aspiration using a pigtail catheter left to low suction or water seal is recommended. Strong suction should not be used with a spontaneous pneumothorax because of an often-delayed presentation and, thus, an increased risk of reexpansion pulmonary edema .
Spontaneous pneumothorax is a life-threatening condition in patients with severe underlying lung disease; thus, tube thoracostomy is the procedure of choice in SSP.
Pleurodesis decreases the risk of recurrence, as does thoracotomy or VATS to excise the bullae.
Iatrogenic and traumatic pneumothorax
Aspiration is the technique of choice for iatrogenic pneumothoraces, because recurrence is usually not a factor. Tube thoracostomy is reserved for very symptomatic patients.
In general, traumatic pneumothorax should be treated with a chest tube, particularly if the patient cannot be closely observed. Chest tubes are attached to a one-way valve apparatus that uses a water chamber to avoid a direct connection to atmospheric pressure (so that during inspiration, when negative pressure is generated, air does not rush into the pleural space), allowing continuous removal of air from the pleural cavity during respiration. Changing the pressure above the water seal allows below-atmospheric suction for further air removal. The collapsed lung reexpands and heals, preventing continued air leakage. After air leaks have ceased for 24 hours, the vacuum may be decreased and the tube removed.
The process of lung reexpansion and healing is not immediate and may be complicated by pulmonary edema; therefore, a chest tube is usually left in place until the clinical conditions are met; any complications warrant longer placement.
A subset of patients who have a small (< 15-20%), minimally symptomatic pneumothorax may be admitted, observed closely, and monitored by using serial chest radiographs. In these patients, administration of 100% oxygen promotes resolution by speeding the absorption of gas from the pleural cavity into the pulmonary vasculature.
Although commonly used, few data exist in the medical literature showing the efficacy of the procedure or reviewing the field-use and incidence of the needle decompression.
Tension pneumothorax
Tension pneumothorax remains a life-threatening condition diagnosed under difficult conditions, with a simple emergency procedure as treatment (ie, needle decompression). Make sure no contraindications exist for the placement of an emergency decompression catheter into the thorax. Previous thoracotomy, previous pneumonectomy, and presence of a coagulation disorder, for example, are relative contraindications, because failure to treat tension pneumothorax expectantly can result in patient death.
In emergency circumstances, decompression catheters should be placed in the second intercostal space in the midclavicular line. This site was confirmed in a review of 100 thoracic CT scans by measuring the distance from the midline to the internal mammary artery (IMA) and the average thickness of the tissues. This procedure punctures the skin and, possibly, the pectoralis major, external intercostals, internal intercostals, and parietal pleura. Placement in the middle third of the clavicle minimizes the risk of injury to the IMA during the procedure. The catheter should be placed just above the cephalad border of the rib; the intercostal vessels are largest on the lower edge of the rib.
Harcke et al had similar results when they used CT analysis of deployed male military personnel to determine that the mean horizontal thickness at the second right intercostal space in the midclavicular line was 5.36 cm and that an 8-cm angiocatheter would reach the pleural space in 99% of the soldiers in this series.
Unfortunately, in a 2005 study of emergency physicians, 21 of whom had completed advanced trauma and life support (ATLS) training, only 60% were able to correctly identify the second intercostal space when attempting to locate the needle thoracostomy site on a human volunteer, and all placed the thoracocentesis needle medial to the midclavicular line. In the same study, 8% of participants inappropriately identified the site used for needle pericardiocentesis, and 4% inappropriately identified the fifth intercostal space in the anterior axillary line.
A 2011 study by Sanchez et al suggested that the anterior approach is typically more successful than the lateral approach when it comes to angiocatheters, though the anterior approach is not failsafe. Further, longer angiocatheters may increase the chances of decompression, but the risk of damage to surrounding vital structures is higher.
In relation to the development of apparent life-threatening hemorrhage after decompression in the second intercostal space at the anterior midclavicular line in patients with no initial evidence of hemothorax on presentation, it has been suggested that a potentially safer option is to decompress a pneumothorax in the fifth intercostal space at the anterior axillary line, similarly to recommendations for chest drain insertion.
If a hemothorax is associated with the pneumothorax, additional chest tubes may be needed to assist drainage of blood and clots. If the hemopneumothorax requires insertion of a second chest tube, the second tube should be directed inferiorly and should be posterior to the apex of the diaphragm.
Another point to take note of is that a significant number of patients have a larger chest wall than can be penetrated by a catheter length of 5 cm. In particular, men undergoing treatment for tension pneumothorax are more likely to have a larger body habitus with wider chest wall, so that performing needle thoracostomy may necessitate the use of a catheter longer than 5 cm to reliably penetrate into the pleural space.
In one study, a catheter length of patients at an American level 1 trauma center showed that a catheter length of 5 cm would reliably penetrate the pleural space in only 75% of patients. A 2008 study analyzing average chest-wall thickness at the second intercostal space in the midclavicular line concluded that a 4.5-cm catheter length may not penetrate the chest wall in approximately 10-35% of trauma patients, depending on age and sex.
Catamenial pneumothorax
Oral contraceptives carry a high success rate in the treatment of catamenial pneumothorax, though this condition may also (rarely) be treated surgically. Most cases present during or shortly after menses, and the spontaneous pneumothorax is usually right-sided.
Pneumomediastinum
Most patients with pneumomediastinum should be observed for signs of serious complications (eg, pneumothorax, tension pneumothorax, mediastinitis). If the pneumomediastinum occurred from the inhalation of cocaine or smoking of marijuana, observation in the ED for progression may be indicated.
A follow-up chest radiograph should be obtained in 12-24 hours to detect any progression or complication, such as pneumothorax. If no progression occurs at 24 hours and if no evidence of mediastinitis exists, the patient may be discharged.
Indications for Surgical Assistance
If the patient has had repeated episodes of pneumothorax or if the lung remains unexpanded after 5 days with a chest tube in place, operative therapy may be necessary. The surgeon may use treatment options such as thoracoscopy, electrocautery, laser treatment, resection of blebs or pleura, or open thoracotomy. Other surgical indications are as follows:
- Persistent air leak for longer than 7 days
- Recurrent ipsilateral pneumothorax
- Contralateral pneumothorax
- Bilateral pneumothorax
- First-time presentation in a patient with a high-risk occupation (eg, diver, pilot)
- Patients with AIDS (often because of extensive underlying necrosis)
- Unacceptable risk of recurrent pneumothorax for patients with plans for extended stays at remote sites
- Lymphangiomyomatosis, a condition causing a high risk of pneumothorax
Video-Assisted Thoracoscopic Surgery
VATS is chosen for recurrent PSP or SSP, particularly for pediatric patients, in whom it has been shown to have better outcomes and shorter recovery. VATS is an alternative to thoracotomy and is performed with the patient under general anesthesia using a camera and small trocar access ports. Other indications include an unexpanded lung 5 days after tube thoracostomy, bronchopleural fistula persisting for 5 days or longer, recurrent pneumothorax after chemical pleurodesis, and occupational reasons (eg, airplane pilots, deep-sea divers).
In a meta-analysis of 12 trials that randomized 670 patients, VATS was associated with shorter length of stay (reduction, 1.0-4.2 d) and less pain or use of pain medication than thoracotomy in the five of seven trials in which the technique was used for pneumothorax or minor lung resection. In the treatment of pneumothorax, VATS was associated with substantially fewer recurrences than pleural drainage in two trials. VATS with resection of large bullous lesions is associated with a recurrence rate of 2-14%.
Thoracotomy
Whereas thoracotomy has been the criterion standard, it is increasingly being replaced by VATS in the treatment of chronic or persisting pneumothoraces, for the aforementioned reasons. Recurrence rates with thoracotomy are as low as 4%.
Talc has been the preferred agent for pleurodesis (see below). It can be administered by insufflation or as a slurry. Insufflation of talc and thoracotomy has had a recurrence rate of 0-7%.
Pleurodesis
In patients with repeated pneumothoraces who are not good candidates for surgery, pleurodesis (or sclerotherapy) may be necessary. Pleurodesis decreases the chance of pneumothorax recurrence and should be performed in consultation with the surgeon. This procedure should be performed just after reinflation of the lung if the presence of an air leak is not a contraindication. The two major sclerosing agents are talc and tetracycline derivatives (eg, minocycline, doxycycline).
Talc (5-10 g in 250 mL sterile isotonic sodium chloride solution) is usually insufflated during VATS or thoracotomy, but one study of 32 patients demonstrated findings of successful treatment with a chest tube (10% recurrence at 5 years).
In a large Department of Veterans Affairs study, tetracycline pleurodesis had a 25% recurrence rate, compared with 41% in control subjects. However, tetracycline no longer is available for pleurodesis, because of stringent manufacturing requirements. Nonetheless, its derivatives minocycline and doxycycline have been shown to be successful sclerosing agents. Bleomycin was found to be ineffective in rabbits and is expensive.
Pleurodesis is painful, and the patient should be premedicated with benzodiazepine and intrapleural lidocaine
Complications
Misdiagnosis is the most common complication of needle decompression. If a pneumothorax but not a tension pneumothorax is present, needle decompression creates an open pneumothorax. Alternatively, if no pneumothorax exists, the patient may develop a pneumothorax after the needle decompression is performed. Additionally, the needle may create a lung laceration, which, though rare, can cause significant pulmonary injury or hemothorax. If the needle is initially placed too medial to the sternum, needle decompression may cause a hemothorax by lacerating the inferior set of intercostal vessels or the internal mammary artery.
Damage to the intercostal neurovascular bundle and lung parenchymal injury can occur following thoracostomy tube placement, especially if trocars are used for placement, and there is an increased risk of postoperative bleeding after lung transplantation for medical pleurodesis and surgery (though length of hospital stay is not affected).
Accidental disconnection and malpositioning of Heimlich valves can complicate an attempted outpatient treatment of pneumothorax via pigtail catheter.
Pneumothorax complications include the following:
- Hypoxemic respiratory failure
- Respiratory or cardiac arrest
- Hemopneumothorax
- Bronchopulmonary fistula
- Pulmonary edema (following lung reexpansion)
- Empyema
- Pneumomediastinum
- Pneumopericardium
- Pneumoperitoneum
- Pyopneumothorax
Complications of surgical procedures include the following:
- Failure to cure the problem
- Acute respiratory distress or failure
- Infection of the pleural space
- Cutaneous or systemic infection
- Persistent air leak
- Reexpansion pulmonary edema
- Pain at the site of chest tube insertion
- Prolonged tube drainage and hospital stay
Reexpansion pulmonary edema
Reexpansion pulmonary edema is a unilateral pulmonary edema that is seen after reinflation of a collapsed lung. It can also occur in the opposite lung. The incidence, etiology, risks, and mortality rates of this condition are controversial.
Findings from animal studies and several case reports in humans indicate that reexpansion pulmonary edema may occur more often if a pneumothorax is present for longer than 3 days, if the evacuation volume is greater than 2000 mL, and if suction is applied. This information is important because in one study, 46% of patients waited more than 2 days after their symptoms started to seek medical attention, and, in another study, 18% waited more than 7 days.
Tension pneumothorax
A worsening pneumothorax, usually with a one-way valve phenomenon, can allow air into the intrapleural space and prevent its escape, causing mediastinal shift, pulmonary shunting, and circulatory collapse.
Treatment of tension pneumothorax is done on an emergency basis and should be performed before confirmatory radiologic studies. Needle decompression is performed before definitive treatment with tube thoracostomy .
In mechanically ventilated patients, high pressures and air trapping place patients at risk for tension pneumothorax if the thoracostomy is not functioning. Patients with smaller pneumothoraces that would otherwise be managed with aspiration or observation sometimes undergo thoracostomy because of the need for mechanical ventilation.
Prevention
Prevention of recurrence
Strategies for the prevention of recurrent pneumothorax include observation, surgical and nonsurgical pleurodesis, and bleb resection. Other important points to keep in mind include the following:
- Prompt recognition and treatment of bronchopulmonary infections decrease the risk of progression to a pneumothorax
- If subclavian vein cannulation is required, the supraclavicular approach should be used rather than the infraclavicular approach whenever possible to help decrease the likelihood of pneumothorax formation
- The incidence of iatrogenic tension pneumothorax may be decreased with prophylactic insertion of a chest tube in patients with a simple pneumothorax that requires positive pressure ventilation
- Pleurodesis decreases the risk of recurrence of spontaneous pneumothorax, as does thoracotomy or VATS to excise the bullae
A study by Chen et al found that pleural abrasion with minocycline pleurodesis was as effective as apical pleurectomy for patients with PSP with high recurrence risk. The two procedures were found to be similar with respect to duration of postoperative chest drainage, length of hospital stay, complication rates, long-term residual chest pain, and long-term pulmonary function. The rate of recurrence was 3.8% for both procedures.
An Italian study reported on a fibrin sealant that was found to be safe and effective for preventing alveolar air leaks after lung resections. The sealant also reportedly shortened the duration of postoperative alveolar air leaks.
A Japanese study found that the use of an absorbable polyglycolic acid sheet to cover the staple line after thoracoscopic bullectomy may prevent the recurrence of PSP.
Observation
Observation is appropriate for iatrogenic pneumothorax in an individual with normal lungs who has responded to treatment with observation or simple aspiration. Simple aspiration or chest tube drainage of pneumothorax does not prevent recurrence. In fact, recurrences have been reported to occur in as many as 32% of PSPs.
One study showed that a Heimlich valve with a small-caliber catheter was less effective in preventing recurrence than closed thoracostomy. In another study, the recurrence rate after 1 year with a Heimlich valve did not differ significantly from that with chest tube placement. Recurrent spontaneous pneumothorax requires more definitive treatment to prevent recurrence. Recurrence rates are higher with SSP than with PSP; hence, observation is less often chosen as an approach in SSP.
Surgical pleurodesis
A patient treated with surgical pleurodesis has a recurrence prevention rate exceeding 90%. Practice variation depends on local practitioner experience, resources, and success with approaches ranging from VATS to surgical thoracotomy and pleurectomy.
Nonsurgical pleurodesis
“Medical” thoracoscopy requires only local anesthesia or conscious sedation, in an endoscopy suite, using nondisposable rigid instruments. Thus, this procedure is considerably less invasive and less expensive, but it is also less effective, particularly in inexperienced hands. Patient comorbidity plays a role in selection of appropriate intervention. The main diagnostic and therapeutic indications for medical thoracoscopy are pleural effusions and pneumothorax.
Success rates for chemical sclerosing agents are as high as 91%, compared with 95-100% for surgical techniques. In an early study, chemical pleurodesis resulted in a significant reduction of recurrence as compared with chest tube drainage alone. Chemical pleurodesis and surgery were equally effective and were both superior to conservative therapy in preventing the recurrence of pneumothorax in LAM.
Consultations
Physicians from various services may be needed to care for patients who require tube thoracostomy, pleurodesis, or surgical thoracotomy and admission. A surgeon and a pulmonologist should evaluate patients underlying lung disease or with recurrent disease to determine the cause and further management.
Treatment of tension pneumothorax should commence immediately after diagnosis, without delay for further consultation and/or evaluation.
A trauma or general surgeon should evaluate patients with trauma, and the patient is often admitted for observation.
Long-Term Monitoring
When a patient is on PPV and normal respiratory function is preserved, routinely follow up decompressed tension pneumothoraces by watching for recurrence of the condition. Chest radiography is helpful but not required.
Direct patients indicating a readiness to quit smoking to their primary care physician or offer referral for cessation management. This may include nicotine replacement and non-nicotine pharmacotherapy (eg, bupropion or varenicline).
Patients should receive follow-up care from a pulmonary physician within 7-10 days.
BTS Guideline for Spontaneous Pneumothorax
In 2023, the British Thoracic Society published a guideline for management of pleural disease, which included the following recommendations for spontaneous pneumothorax.
Acute management
For treatment of minimally symptomatic (ie, no significant pain or breathlessness and no physiologic compromise) or asymptomatic primary spontaneous pneumothorax (PSP) in adults, regardless of size, conservative management can be considered.
For initial treatment of PSP in adults who have good support and are being treated in centers with sufficient expertise and follow-up facilities available, ambulatory management should be considered.
For initial treatment of adults with PSP who are not deemed suitable for conservative or ambulatory management, needle aspiration or tube drainage should be considered.
For prevention of recurrent or secondary spontaneous pneumothorax (SSP) in adults (eg, patients with severe chronic obstructive pulmonary disease who significantly decompensated in the presence of a pneumothorax, even during or after the first episode), chemical pleurodesis can be considered.
For treatment of pneumothorax in adults at initial presentation if prevention of recurrence is a priority is deemed important (eg, in patients who present with tension pneumothorax who work in high-risk occupations), thoracic surgery can be considered.
Optimal approach and operation for surgical management
In the general management of pneumothorax in adults, video-assisted thoracoscopy access can be considered for surgical pleurodesis.
For the lowest level of recurrence risk required for specific (eg, high-risk) occupations, thoracotomy access and surgical pleurodesis should be considered.
For the treatment of spontaneous pneumothorax in adults, surgical pleurodesis and/or bullectomy should be considered.
Medication Summary
A tension pneumothorax requires treatment with rapidity. However, anesthetics and analgesics should be used if the patient is not in distress. The goals of pharmacotherapy are to reduce morbidity and to prevent complications. In addition to the medications discussed in this section, talc may be used as a sclerosing agent for pleurodesis by mixing 2-5 g in 250 mL of sterile isotonic sodium chloride solution to form a slurry or poudrage. Note that acute respiratory distress syndrome (ARDS) has been reported after use of talc as a pleural sclerosing agent, but this is considered a rare complication.
Local Anesthetics
Local anesthetic agents are used for analgesia for thoracentesis and chest tube placement.
Lidocaine hydrochloride (Xylocaine, LidaMantle, Anestacon)
Lidocaine hydrochloride is a local anesthetic that may be absorbed following topical administration to mucous membranes. Its rate and extent of absorption depends on the specific site of application, duration of exposure, concentration, and total dosage. This drug acts by decreasing the permeability to sodium ions in neuronal membranes, resulting in the inhibition of depolarization, and blocking the transmission of nerve impulses. Adverse effects with the use of lidocaine hydrochloride as a local anesthetic include allergic reactions.
Opiate Analgesics
Opiate analgesic agents are used for pain control, which is essential to good patient care, ensures patient comfort, and promotes pulmonary toilet. Most analgesics have sedating properties, which are beneficial for patients with painful skin lesions. These drugs are important in the initial placement of thoracostomy tubes and for controlling pain after the procedure.
Fentanyl citrate (Sublimaze)
The onset of analgesia with fentanyl citrate is immediate with intravenous (IV) administration, and the duration of analgesia is 30-60 minutes. However, the respiratory depressant effect may last longer than analgesia. The dose should be individualized, and vital signs should be monitored in routinely.
Morphine (Astramorph, Infumorph 200, MS Contin, Oramorph SR)
Morphine is the drug of choice for analgesia because of its reliable and predictable effects, safety profile, and ease of reversibility with naloxone.
In adults, the initial dose is given intravenously and titrated for effect. Recommended doses begin at 0.1 mg/kg, but practically the regimen is 1-2 mg IV. The dose may be repeated at frequent intervals until analgesia is reached, and then the interval is lengthened. For most adults, the usual upper limit during the acute event is 10-15 mg over the first 4 hours. Morphine IV, delivered via patient-controlled analgesia machines, is set to get 1-2 mg on demand every 6 minutes, with a 4-hour lockout of 30 mg. Conversion to oral narcotics is accomplished as soon as possible.
In neonates, the dose is 0.05-0.2 mg/kg IV/IM/SC as needed; in children, the dose is 0.1-0.2 mg/kg IV/IM/SC q2-4h as needed. As in adults, the IV doses vary and the drug should be titrated for the desired effect.
Benzodiazepines
Benzodiazepines are used for conscious sedation. These agents are useful for premedication before pleurodesis/sclerotherapy or placement of a thoracostomy tube.
Midazolam
Benzodiazepine used for sedation component of conscious sedation protocol. Onset of action occurs within 1-5 min. Half-life of 1-4 h. Prolonged with liver cirrhosis, congestive heart failure, obesity, and old age.
Lorazepam (Ativan)
Lorazepam is a sedative hypnotic with a short onset of effects and a relatively long half-life. This drug acts by increasing the action of gamma aminobutyric acid (GABA), a major inhibitory neurotransmitter in the brain. However, lorazepam may depress all levels of the central nervous system (CNS), including the limbic and reticular formations.
The initial adult dose is 2 mg total or 0.044 mg/kg IV, whichever is smaller. Alternatively, administer 0.05 mg/kg IV, not to exceed 4 mg/dose.
In children, the dose is 0.05-0.1 mg/kg IV slowly over 2-5 minutes; a slow 0.5 mg/kg IV dose may be repeated.
Antibiotics
In patients with repeated pneumothoraces who are not good candidates for surgery, pleurodesis (or sclerotherapy) may be necessary. Two major sclerosing agents that can be used are talc and doxycycline. Prophylactic antibiotics are not recommended for the placement of chest tubes in nontraumatic causes.
Doxycycline (Vibramycin, Vibra-Tabs, Doryx)
Doxycycline is broad-spectrum, synthetically derived bacteriostatic antibiotic in the tetracycline class. It is almost completely absorbed, concentrates in bile, and is excreted in urine and feces as a biologically active metabolite in high concentrations.
Cefazolin (Kefzol)
Cefazolin is a first-generation cephalosporin. According to the Eastern Association for the Surgery of Trauma (EAST) Practice guidelines, a first-generation cephalosporin should be administered for no longer than 24 hours after tube thoracostomy.