Practice Essentials
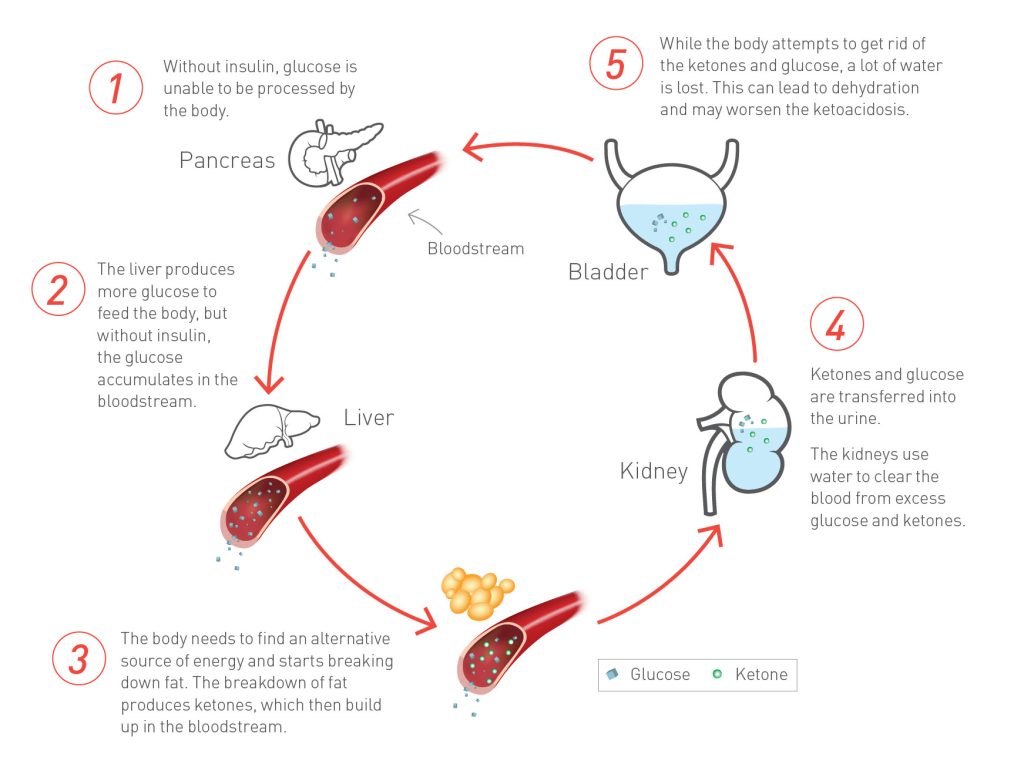
Diabetic ketoacidosis (DKA) is an acute, major, life-threatening complication of diabetes characterized by hyperglycemia, ketoacidosis, and ketonuria. It occurs when absolute or relative insulin deficiency inhibits the ability of glucose to enter cells for utilization as metabolic fuel, the result being that the liver rapidly breaks down fat into ketones to employ as a fuel source. The overproduction of ketones ensues, causing them to accumulate in the blood and urine and turn the blood acidic. DKA occurs mainly in patients with type 1 diabetes, but it is not uncommon in some patients with type 2 diabetes. Laboratory studies for DKA include glucose blood tests, serum electrolyte determinations, blood urea nitrogen (BUN) evaluation, and arterial blood gas (ABG) measurements. Treatment includes correction of fluid loss with intravenous fluids; correction of hyperglycemia with insulin; correction of electrolyte disturbances, particularly potassium loss; correction of acid-base balance; and management of concurrent infection (if present).
Signs and symptoms of diabetic ketoacidosis
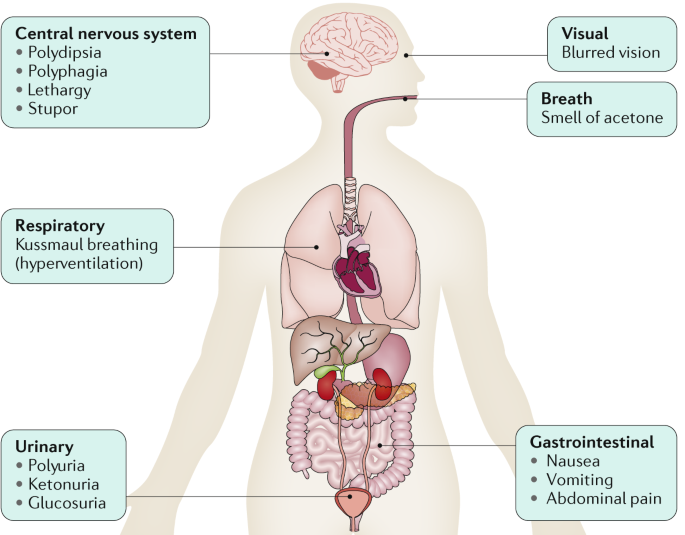
The most common early symptoms of DKA are the insidious increase in polydipsia and polyuria. The following are other signs and symptoms of DKA:
Malaise, generalized weakness, and fatigability
Nausea and vomiting; may be associated with diffuse abdominal pain, decreased appetite, and anorexia
Rapid weight loss in patients newly diagnosed with type 1 diabetes
History of failure to comply with insulin therapy or missed insulin injections due to vomiting or psychological reasons or history of mechanical failure of insulin infusion pump
Decreased perspiration
Altered consciousness (eg, mild disorientation, confusion); frank coma is uncommon but may occur when the condition is neglected or with severe dehydration/acidosis

Signs and symptoms of DKA associated with possible intercurrent infection are as follows:
Fever
Coughing
Chills
Chest pain
Dyspnea
Arthralgia
General findings in diabetic ketoacidosis
On examination, general findings of DKA may include the following:
Ill appearance
Dry skin
Labored respiration
Dry mucous membranes
Decreased skin turgor
Decreased reflexes
Characteristic acetone (ketotic) breath odor
Tachycardia
Hypotension
Tachypnea
Hypothermia
In addition, evaluate patients for signs of possible intercurrent illnesses such as MI, UTI, pneumonia, and perinephric abscess. Search for signs of infection is mandatory in all cases.
Laboratory studies
Initial and repeat laboratory studies for patients with DKA include the following:
Serum glucose levels
Serum electrolyte levels (eg, potassium, sodium, chloride, magnesium, calcium, phosphorus)
Bicarbonate levels
Amylase and lipase levels
Urine dipstick
Ketone levels
Serum or capillary beta-hydroxybutyrate levels
ABG measurements
Complete blood count (CBC)
BUN and creatinine levels
Urine and blood cultures if intercurrent infection is suspected
Electrocardiogram (ECG; or telemetry in patients with comorbidities)
Note that high serum glucose levels may lead to dilutional hyponatremia; high triglyceride levels may lead to factitious low glucose levels; and high levels of ketone bodies may lead to factitious elevation of creatinine levels.
Imaging studies
Radiologic studies that may be helpful in patients with DKA include the following:
Chest radiography – To rule out pulmonary infection such as pneumonia
Head computed tomography (CT) scanning – To detect early cerebral edema; use low threshold in children with DKA and altered mental status
Head magnetic resonance imaging (MRI) – To detect early cerebral edema (order only if altered consciousness is present )
Do not delay administration of hypertonic saline or mannitol in those pediatric cases where cerebral edema is suspected, as many changes may be seen late on head imaging.
Management goals
Treatment of ketoacidosis should aim for the following:
Fluid resuscitation
Reversal of the acidosis and ketosis
Reduction in the plasma glucose concentration to normal
Replenishment of electrolyte and volume losses
Identification the underlying cause
Pharmacotherapy
Regular and analog human insulins are used for correction of hyperglycemia, unless bovine or pork insulin is the only available insulin.
Medications used in the management of DKA include the following:
Rapid-acting insulins (eg, insulin aspart, insulin glulisine, insulin lispro)
Short-acting insulins (eg, regular insulin)
Electrolyte supplements (eg, potassium chloride)
Alkalinizing agents (eg, sodium bicarbonate)
Background
Diabetic ketoacidosis (DKA) is an acute, major, life-threatening complication of diabetes. DKA mainly occurs in patients with type 1 diabetes, but it is not uncommon in some patients with type 2 diabetes (most likely latent autoimmune diabetes of adults [LADA] or Flatbush diabetes).
DKA is a state of absolute or relative insulin deficiency aggravated by ensuing hyperglycemia, dehydration, and acidosis-producing derangements in intermediary metabolism. The most common causes are underlying infection, disruption of insulin treatment, and new onset of diabetes.
DKA is defined clinically as an acute state of severe uncontrolled diabetes associated with ketoacidosis that requires emergency treatment with insulin and intravenous fluids.
Biochemically, DKA is defined as an increase in the serum concentration of ketones greater than 5 mEq/L, a blood sugar level greater than 250 mg/dL (although it is usually much higher), and a blood (usually arterial) pH less than 7.3. Ketonemia and ketonuria are characteristic, as is a serum bicarbonate level of 18 mEq/L or less (less than 5 mEq/L is indicative of severe DKA). These biochemical changes are frequently associated with increased anion gap, increased serum osmolarity and increased serum uric acid.
Herrington et al collected simultaneous arterial and venous samples from 206 critically ill patients and analyzed in duplicate. [3] They calculated coefficients of variation and 95% limits of agreement for arterial and venous samples and constructed statistical plots to assess the degree of agreement between samples. They found that coefficients of variation for arterial and venous samples were similar for pH, serum bicarbonate, and potassium, indicating that both are sufficiently reliable for the management of critically ill patients, particularly those with DKA.
Mental status changes can be seen with mild-to-moderate DKA; more severe deterioration in mental status is typical with moderate-to-severe DKA.
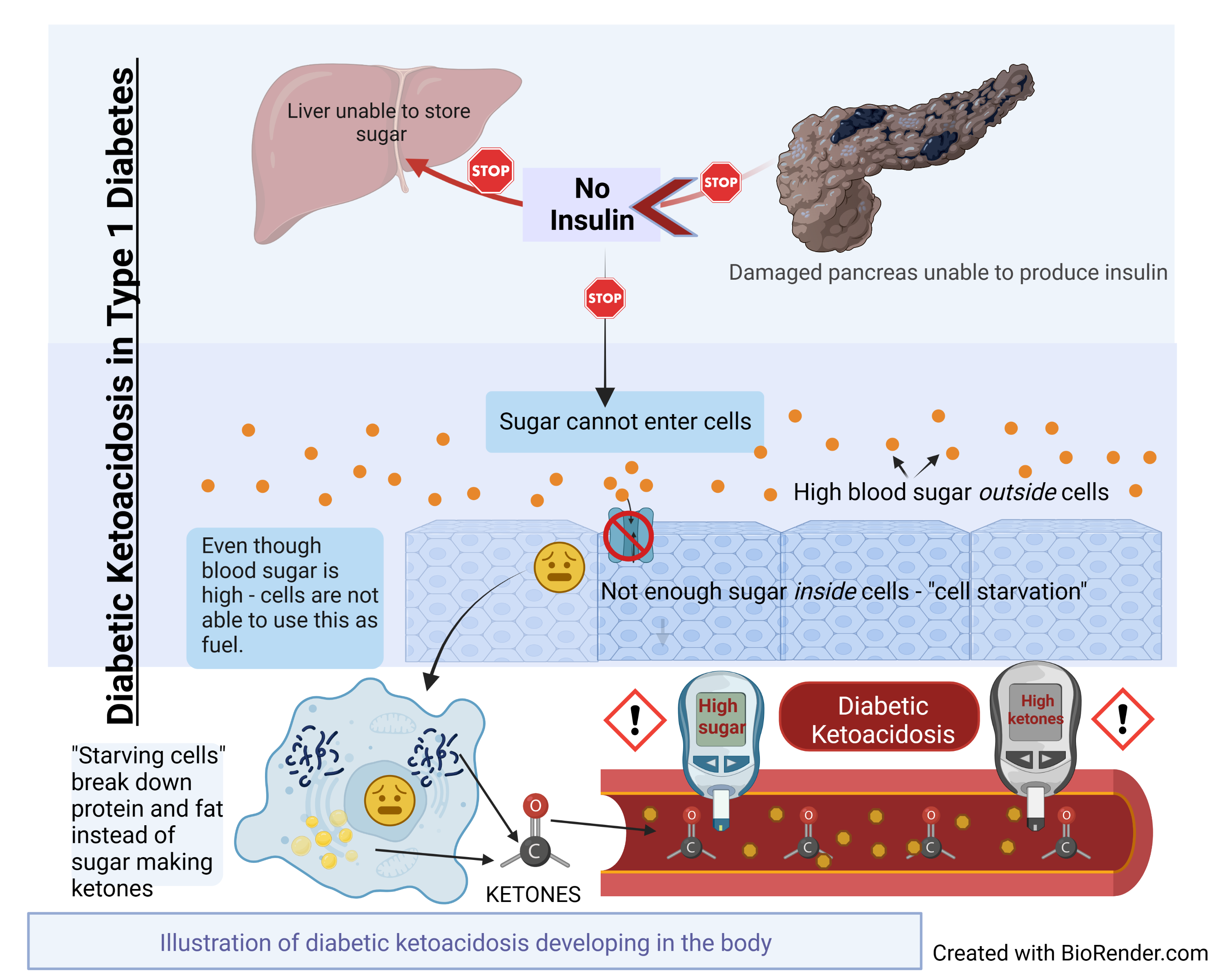
Pathophysiology
Diabetic ketoacidosis (DKA) is a complex disordered metabolic state characterized by hyperglycemia, ketoacidosis, and ketonuria. DKA usually occurs as a consequence of absolute or relative insulin deficiency that is accompanied by an increase in counter-regulatory hormones (ie, glucagon, cortisol, growth hormone, epinephrine). This type of hormonal imbalance enhances hepatic gluconeogenesis, glycogenolysis, and lipolysis.
Hepatic gluconeogenesis, glycogenolysis secondary to insulin deficiency, and counter-regulatory hormone excess result in severe hyperglycemia, while lipolysis increases serum free fatty acids. Hepatic metabolism of free fatty acids as an alternative energy source (ie, ketogenesis) results in accumulation of acidic intermediate and end metabolites (ie, ketones, ketoacids). Ketone bodies have generally included acetone, beta-hydroxybutyrate, and acetoacetate. It should be noted, however, that only acetone is a true ketone, while acetoacetic acid is true ketoacid and beta-hydroxybutyrate is a hydroxy acid.
Meanwhile, increased proteolysis and decreased protein synthesis as result of insulin deficiency add more gluconeogenic substrates to the gluconeogenesis process. In addition, the decreased glucose uptake by peripheral tissues due to insulin deficiency and increased counter regulatory hormones increases hyperglycemia.
Ketone bodies are produced from acetyl coenzyme A mainly in the mitochondria within hepatocytes when carbohydrate utilization is impaired because of relative or absolute insulin deficiency, such that energy must be obtained from fatty acid metabolism. High levels of acetyl coenzyme A present in the cell inhibit the pyruvate dehydrogenase complex, but pyruvate carboxylase is activated. Thus, the oxaloacetate generated enters gluconeogenesis rather than the citric acid cycle, as the latter is also inhibited by the elevated level of nicotinamide adenine dinucleotide (NADH) resulting from excessive beta-oxidation of fatty acids, another consequence of insulin resistance/insulin deficiency. The excess acetyl coenzyme A is therefore rerouted to ketogenesis.
Progressive rise of blood concentration of these acidic organic substances initially leads to a state of ketonemia, although extracellular and intracellular body buffers can limit ketonemia in its early stages, as reflected by a normal arterial pH associated with a base deficit and a mild anion gap.
When the accumulated ketones exceed the body’s capacity to extract them, they overflow into urine (ie, ketonuria). If the situation is not treated promptly, a greater accumulation of organic acids leads to frank clinical metabolic acidosis (ie, ketoacidosis), with a significant drop in pH and bicarbonate serum levels. Respiratory compensation for this acidotic condition results in Kussmaul respirations, ie, rapid, shallow breathing (sigh breathing) that, as the acidosis grows more severe, becomes slower, deeper, and labored (air hunger).
Ketones/ketoacids/hydroxy acids, in particular, beta-hydroxybutyrate, induce nausea and vomiting that consequently aggravate fluid and electrolyte loss already existing in DKA. Moreover, acetone produces the fruity breath odor that is characteristic of ketotic patients.
Glucosuria leads to osmotic diuresis, dehydration and hyperosmolarity. Severe dehydration, if not properly compensated, may lead to impaired renal function. Hyperglycemia, osmotic diuresis, serum hyperosmolarity, and metabolic acidosis result in severe electrolyte disturbances. The most characteristic disturbance is total body potassium loss. This loss is not mirrored in serum potassium levels, which may be low, within the reference range, or even high.
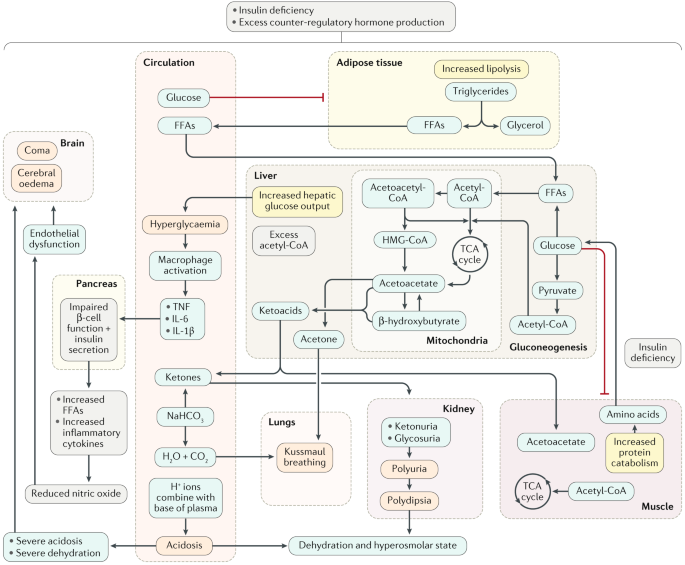
Potassium loss is caused by a shift of potassium from the intracellular to the extracellular space in an exchange with hydrogen ions that accumulate extracellularly in acidosis. Much of the shifted extracellular potassium is lost in urine because of osmotic diuresis.
Patients with initial hypokalemia are considered to have severe and serious total body potassium depletion. High serum osmolarity also drives water from intracellular to extracellular space, causing dilutional hyponatremia. Sodium also is lost in the urine during the osmotic diuresis.
Typical overall electrolyte loss includes 200-500 mEq/L of potassium, 300-700 mEq/L of sodium, and 350-500 mEq/L of chloride. The combined effects of serum hyperosmolarity, dehydration, and acidosis result in increased osmolarity in brain cells that clinically manifests as an alteration in the level of consciousness.
Many of the underlying pathophysiologic disturbances in DKA are directly measurable by the clinician and need to be monitored throughout the course of treatment. Close attention to clinical laboratory data allows for tracking of the underlying acidosis and hyperglycemia, as well as prevention of common potentially lethal complications such as hypoglycemia, hyponatremia, and hypokalemia.
Hyperglycemia
The absence of insulin, the primary anabolic hormone, means that tissues such as muscle, fat, and liver do not uptake glucose. Counterregulatory hormones, such as glucagon, growth hormone, and catecholamines, enhance triglyceride breakdown into free fatty acids and gluconeogenesis, which is the main cause for the elevation in serum glucose level in DKA. Beta-oxidation of these free fatty acids leads to increased formation of ketone bodies.
Overall, metabolism in DKA shifts from the normal fed state characterized by carbohydrate metabolism to a starvation state characterized by fat metabolism.
Secondary consequences of the primary metabolic derangements in DKA include an ensuing metabolic acidosis as the ketone bodies produced by beta-oxidation of free fatty acids deplete extracellular and cellular acid buffers. The hyperglycemia-induced osmotic diuresis depletes sodium, potassium, phosphates, and water.
Hyperglycemia usually exceeds the renal threshold of glucose absorption and results in significant glucosuria. Consequently, water loss in the urine is increased due to osmotic diuresis induced by glucosuria. This incidence of increased water loss results in severe dehydration, thirst, tissue hypoperfusion, and, possibly, lactic acidosis, or renal impairment.
Dehydration and electrolyte loss
Typical free water loss in DKA is approximately 6 liters or nearly 100 mL/kg of body weight. The initial half of this amount is derived from intracellular fluid and precedes signs of dehydration, while the other half is from extracellular fluid and is responsible for signs of dehydration.
Patients often are profoundly dehydrated and have a significantly depleted potassium level (as high as 5 mEq/kg body weight). A normal or even elevated serum potassium concentration may be seen due to the extracellular shift of potassium in acidotic conditions, and this very poorly reflects the patient’s total potassium stores. The serum potassium concentration can drop precipitously once insulin treatment is started, so great care must be taken to repeatedly monitor serum potassium levels. Urinary loss of ketoanions with brisk diuresis and intact renal function also may lead to a component of hyperchloremic metabolic acidosis.
Etiology
The most common scenarios for diabetic ketoacidosis (DKA) are underlying or concomitant infection (40%), missed or disrupted insulin treatments (25%), and newly diagnosed, previously unknown diabetes (15%). Other associated causes make up roughly 20% in the various scenarios.
Causes of DKA in type 1 diabetes mellitus include the following:
In 25% of patients, DKA is present at diagnosis of type 1 diabetes due to acute insulin deficiency (occurs in 25% of patients)
Poor compliance with insulin through the omission of insulin injections, due to lack of patient/guardian education or as a result of psychological stress, particularly in adolescents
Missed, omitted or forgotten insulin doses due to illness, vomiting or excess alcohol intake
Bacterial infection and intercurrent illness (eg, urinary tract infection [UTI])
Klebsiella pneumoniae (the leading cause of bacterial infections precipitating DKA)
Medical, surgical, or emotional stress
Brittle diabetes
Idiopathic (no identifiable cause)
Insulin infusion catheter blockage
Mechanical failure of the insulin infusion pump
Causes of DKA in type 2 diabetes mellitus include the following:
Intercurrent illness (eg, myocardial infarction, pneumonia, prostatitis, UTI)
Medication (eg, corticosteroids, pentamidine, clozapine)
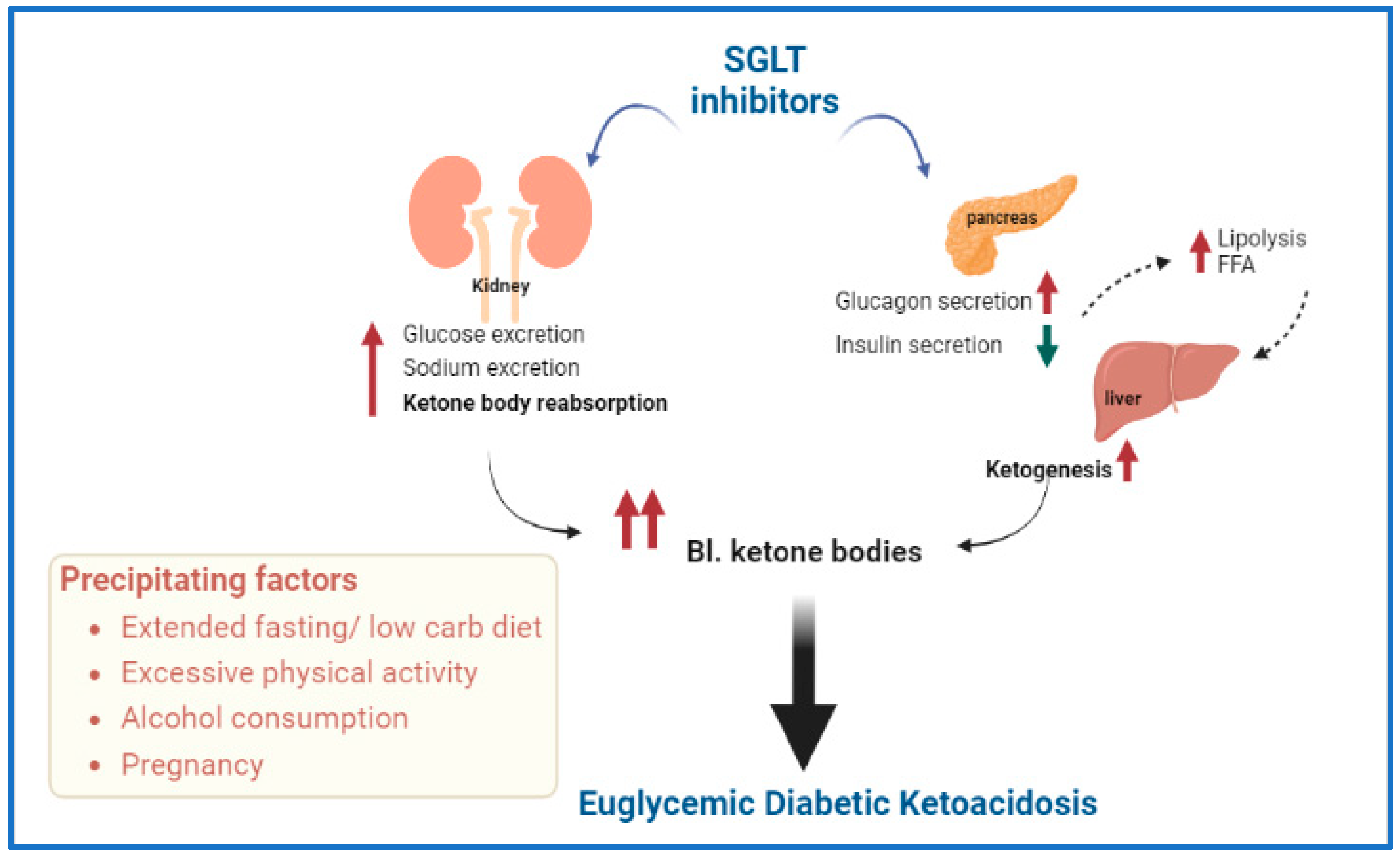
DKA has also been reported in people with type 2 diabetes treated with sodium-glucose cotransporter-2 (SGLT2) inhibitors.
DKA also occurs in pregnant women, either with preexisting diabetes or with diabetes diagnosed during pregnancy. Physiologic changes unique to pregnancy provide a background for the development of DKA. DKA in pregnancy is a medical emergency, as mother and fetus are at risk for morbidity and mortality.
There is evidence that coronavirus disease 2019 (COVID-19) increases the risk of DKA, possibly in association with beta-cell destruction that may result from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19. [8, 9] Moreover, a study by Vitale et al suggested that in persons with type 2 diabetes who are taking SGLT2 inhibitors, the risk of euglycemic DKA may be further increased in the presence of COVID-19 (as based on five cases).
Epidemiology
Despite advancements in self-care of patients with diabetes, DKA accounts for 14% of all hospital admissions of patients with diabetes and 16% of all diabetes-related fatalities. Almost 50% of diabetes-related admissions in young persons are related to DKA. DKA frequently is observed during the diagnosis of type 1 diabetes and often indicates this diagnosis. While the exact incidence is not known, it is estimated to be 1 out of 2000.
DKA occurs primarily in patients with type 1 diabetes. The incidence is roughly 2 episodes per 100 patient years of diabetes, with about 3% of patients with type 1 diabetes initially presenting with DKA. It can occur in patients with type 2 diabetes as well; this is less common, however.
A study by Zhong et al found that in England, for adults with type 1 or type 2 diabetes, there was a growing incidence of hospitalization for DKA between 1998 and 2013. More specifically, the investigators reported that the incidence for patients with type 1 diabetes rose between 1998 and 2007 and then remained at the same level until 2013, while the incidence associated with type 2 diabetes expanded annually by 4.24% between 1998 and 2013.
The incidence of diabetic ketoacidosis in developing countries is not known, but it may be higher than in industrialized nations.
The incidence of DKA is higher in whites because of the higher incidence of type 1 diabetes in this racial group. The incidence of DKA is slightly greater in females than in males for reasons that are unclear. Recurrent DKA frequently is seen in young women with type 1 diabetes and is caused mostly by the omission of insulin treatment.
Among persons with type 1 diabetes, DKA is much more common in young children and adolescents than it is in adults. DKA tends to occur in individuals younger than 19 years, but it may occur in patients with diabetes at any age.
Although multiple factors (eg, ethnic minority, lack of health insurance, lower body mass index, preceding infection, delayed treatment) affect the risk of developing DKA among children and young adults, intervention is possible between symptom onset and development of DKA.
Prognosis
The overall mortality rate for DKA is 0.2-2%, with persons at the highest end of the range residing in developing countries. The presence of deep coma at the time of diagnosis, hypothermia, and oliguria are signs of poor prognosis.
The prognosis of properly treated patients with diabetic ketoacidosis is excellent, especially in younger patients if intercurrent infections are absent. The worst prognosis usually is observed in older patients with severe intercurrent illnesses (eg, myocardial infarction, sepsis, or pneumonia), especially when these patients are treated outside an intensive care unit.
A study by Lee et al reported that in adult patients with DKA, a longer time to resolution was associated with lower pH levels and higher serum potassium concentrations at hospital admission (with both factors being independent predictors).
When DKA is treated properly, it rarely produces residual effects. Before the discovery of insulin in 1922, the mortality rate was 100%. Over the last 3 decades, mortality rates from DKA have markedly decreased in developed countries, from 7.96% to 0.67%.
A fetal mortality rate as high as 30% is associated with DKA. The rate is as high as 60% in diabetic ketoacidosis with coma. Fetal death typically occurs in women with overt diabetes, but it may occur with gestational diabetes. In children younger than 10 years, diabetic ketoacidosis causes 70% of diabetes-related fatalities.
The best results are observed in patients treated in intensive care units during the first 1-2 days of hospitalization, although some hospitals are successful in treating mild cases of DKA in the emergency room (ie, Emergency Valuable Approach and Diabetes Education [EVADE] protocol). A high mortality rate among nonhospitalized patients illustrates the necessity of early diagnosis and implementation of effective prevention programs.
Cerebral edema remains the most common cause of mortality, particularly in young children and adolescents. Cerebral edema frequently results from rapid intracellular fluid shifts. Other causes of mortality include severe hypokalemia, adult respiratory distress syndrome, and comorbid states (eg, pneumonia, acute myocardial infarction).
A heightened understanding of the pathophysiology of DKA along with proper monitoring and correction of electrolytes has resulted in a significant reduction in the overall mortality rate from this life-threatening condition in most developed countries.
A study by Hursh et al indicated that acute kidney injury (AKI) is a frequent development in children hospitalized for DKA. Of 165 hospitalized pediatric DKA patients in the study, AKI developed in 106 (64%). Using an adjusted multinomial logistic regression model, the investigators found a 5-fold increase in the chance of severe AKI (stage 2 or 3) when a patient’s serum bicarbonate level was below 10 mEq/L, while the likelihood of severe AKI rose 22% with each increase in the initial heart rate of five beats/min. The odds of mild AKI (stage 1) developing rose by three fold with an initial corrected sodium level of 145 mEq/L or more.
A study by Chen et al indicated that among persons with type 2 diabetes, those with DKA have a 1.55 times greater risk of stroke than do those without DKA. The stroke risk was particularly high in DKA patients with hypertension and hyperlipidemia and in the first six months after the diagnosis of DKA.
Patient Education
The introduction of diabetes educational programs in most diabetes clinics has contributed to a reduction in the occurrence of diabetic ketoacidosis (DKA) in patients with known diabetes. Such programs teach patients how to avoid DKA by self-testing for urinary ketones when their blood glucose is high or when they have unexplained nausea or vomiting and adjusting their insulin regimens on sick days.
It is essential to educate patients in the prevention of diabetic ketoacidosis (DKA) so that a recurrent episode can be avoided. Central to patient education programs for adults with diabetes is instruction on the self-management process and on how to handle the stress of intercurrent illness.
The patient education program needs to ensure that patients understand the importance of close and careful monitoring of blood sugar levels, particularly during infection, trauma, and other periods of stress.
History
Insidious increased thirst (ie, polydipsia) and urination (ie, polyuria) are the most common early symptoms of diabetic ketoacidosis (DKA). Malaise, generalized weakness, and fatigability also can present as symptoms of DKA.
Nausea and vomiting usually occur and may be associated with diffuse abdominal pain, decreased appetite, and anorexia. A history of rapid weight loss is a symptom in patients who are newly diagnosed with type 1 diabetes.
Patients may present with a history of failure to comply with insulin therapy or missed insulin injections due to vomiting or psychological reasons. Decreased perspiration is another possible symptom of DKA.
Altered consciousness in the form of mild disorientation or confusion can occur. Although frank coma is uncommon, it may occur when the condition is neglected or if dehydration or acidosis is severe.
Among the symptoms of DKA associated with possible intercurrent infection are fever, dysuria, coughing, malaise, chills, chest pain, shortness of breath, and arthralgia. Acute chest pain or palpitation may occur in association with myocardial infarction. Painless infarction is not uncommon in patients with diabetes and should always be suspected in elderly patients.
A study by Crossen et al indicated that in children with type 1 diabetes, those who have had a recent emergency department visit and have undergone a long period without visiting an endocrinologist are more likely to develop DKA. The study included 5263 pediatric patients with type 1 diabetes.
Physical Examination
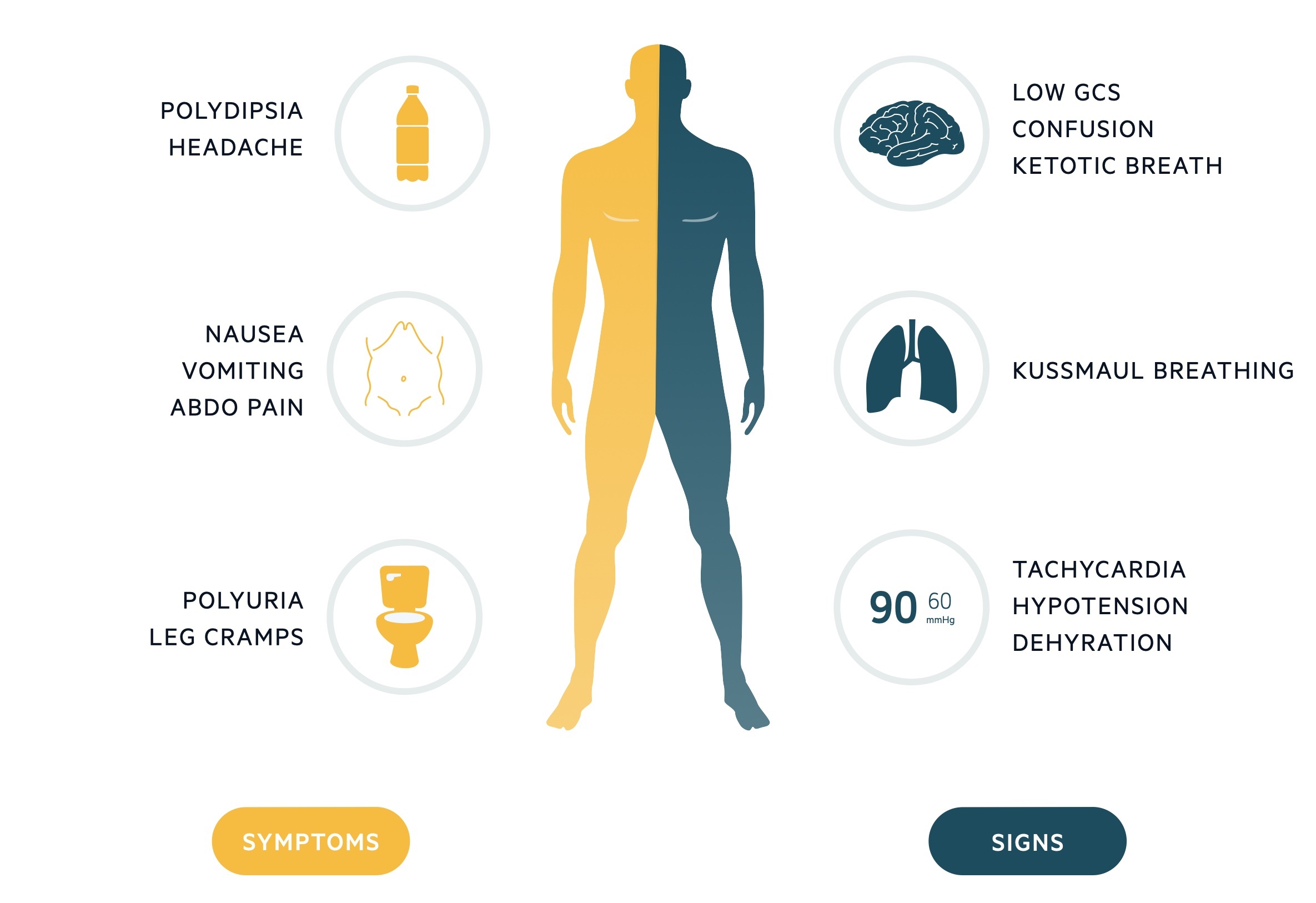
General signs of diabetic ketoacidosis (DKA) may include the following:
Ill appearance
Dry skin
Labored respiration
Dry mucous membranes
Decreased skin turgor
Decreased reflexes
Characteristic acetone (ketotic) breath odor
Effects on vital signs that are related to DKA may include the following:
Tachycardia
Hypotension
Tachypnea
Hypothermia
Fever, if infection is present
Specific signs of DKA may include the following:
Confusion
Coma
Abdominal tenderness
The physical examination should also include detection of the signs of possible intercurrent illnesses such as myocardial infarction, urinary tract infection, pneumonia, and perinephric abscess. Search for signs of infection is mandatory in all cases.
Signs and Symptoms of Hyperglycemia, Acidosis, and Dehydration
Symptoms of hyperglycemia associated with diabetic ketoacidosis may include thirst, polyuria, polydipsia, and nocturia.
Signs of acidosis may include rapid, shallow breathing (sigh breathing) that, as the acidosis grows more severe, becomes slower, deeper, and labored (air hunger), as well as abdominal tenderness and disturbance of consciousness. Although these signs are not usual in all cases of diabetic ketoacidosis (DKA), their presence signifies a severe form of DKA. The breath has a fruity smell.
Signs of dehydration include a weak and rapid pulse, dry tongue and skin, hypotension, and increased capillary refill time.
Emphasizing that no direct correlation exists between the degree of acidosis, hyperglycemia, and the disturbances in the level of consciousness is important.
Complications Associated with DKA
Complications associated with DKA include sepsis and diffuse ischemic processes. Other associated complications include the following:
CVT
Myocardial infarction
DVT
Acute gastric dilatation
Erosive gastritis
Late hypoglycemia
Respiratory distress
Infection (most commonly, urinary tract infections)
Hypophosphatemia
Mucormycosis
Cerebrovascular accident
A prospective study by Jessup et al indicated that in pediatric patients with new-onset type 1 diabetes, those with severe, but uncomplicated, DKA tended to display lower cognitive functioning following correction of the DKA than did age-matched patients without DKA. The investigators suggested that DKA and/or its treatment produces a neuronal insult that causes “acute and possibly long-term cognitive deficits.”
Diagnostic Considerations
In considering a diagnosis of diabetic ketoacidosis (DKA), the following indications should be taken into account: uremia, acute hypoglycemia coma, and catheter-related venous thrombosis, especially with femoral central venous catheters in children.
Differential Diagnoses
Acute Pancreatitis
Alcoholic Ketoacidosis
Appendicitis
Urinary Tract Infection (UTI) and Cystitis (Bladder Infection) in Females
Hyperosmolar Coma
Hypophosphatemia
Hypothermia
Lactic Acidosis
Metabolic Acidosis
Salicylate Toxicity
Septic Shock
Approach Considerations
Diabetic ketoacidosis is typically characterized by hyperglycemia of over 250 mg/dL, a bicarbonate level of less than 18 mEq/L, and a of pH less than 7.30, with ketonemia and ketonuria.
While definitions vary, mild DKA can be categorized by a pH level of 7.25-7.3 and a serum bicarbonate level between 15-18 mEq/L; moderate DKA can be categorized by a pH between 7.0-7.24 and a serum bicarbonate level of 10 to less than 15 mEq/L; and severe DKA has a pH less than 7.0 and bicarbonate less than 10 mEq/L.[23] In mild DKA, anion gap is greater than 10 and in moderate or severe DKA the anion gap is greater than 12. These figures differentiate DKA from HHS where blood glucose is greater than 600 mg/dL but pH is greater than 7.3 and serum bicarbonate greater than 15 mEq/L.
Laboratory studies for diabetic ketoacidosis (DKA) should be scheduled as follows:
Blood tests for glucose every 1-2 h until patient is stable, then every 4-6 h
Serum electrolyte determinations every 1-2 h until patient is stable, then every 4-6 h
Initial blood urea nitrogen (BUN)
Initial arterial blood gas (ABG) measurements, followed with bicarbonate as necessary
Repeat laboratory tests are critical, including potassium, glucose, electrolytes, and, if necessary, phosphorus. Initial workup should include aggressive volume, glucose, and electrolyte management.
It is important to be aware that high serum glucose levels may lead to dilutional hyponatremia; high triglyceride levels may lead to factitious low glucose levels; and high levels of ketone bodies may lead to factitious elevation of creatinine levels.
Plasma Glucose Study
The blood sugar level for patients with DKA usually exceeds 250 mg/dL. The clinician can perform a fingerstick blood glucose test while waiting for the plasma glucose level.
Urine Dipstick Testing
For patients with DKA, the urine dipstick test is highly positive for glucose and ketones. Rarely, urine is negative for ketones, due to the fact that most available laboratory tests can detect only acetoacetate, while the predominant ketone in severe untreated DKA is beta-hydroxybutyrate.
When the clinical condition improves with treatment, the urine test result becomes positive due to the returning predominance of acetoacetate.
Ketones
In patients with DKA, serum ketones are present. Blood beta-hydroxybutyrate levels measured by a reagent strip (Ketostix, N-Multistix, and Labstix) and serum ketone levels assessed by the nitroprusside reaction are equally effective in diagnosing DKA in uncomplicated cases.
The Acetest and Ketostix products measure blood and urine acetone and acetoacetic acid. They do not, however, measure beta-hydroxybutyrate, so the patient may appear to have “paradoxical worsening” as the latter is oxidized to acetoacetate in extrahepatic tissues with improved perfusion and better oxygenation.
Specific testing for beta-hydroxybutyrate can be performed by many laboratories. Diagnosis of ketonuria requires adequate renal function. Additionally, ketonuria may last longer than the underlying tissue acidosis.
One study suggests that routine urine testing for ketones is no longer necessary to diagnose DKA. Using capillary beta hydroxybutyrate offers a distinct advantage of avoiding unnecessary workup.
According to the 2011 Joint British Diabetes Societies (JBDS) guideline for the management of diabetic ketoacidosis, capillary blood ketones should be measured in order to monitor the response to DKA treatment. The method of choice is bedside measurement of blood ketones using a ketone meter. In the absence of blood ketone measurement, venous pH and bicarbonate should be used together with bedside blood glucose monitoring to evaluate treatment response.
Beta-Hydroxybutyrate
Serum or capillary beta-hydroxybutyrate can be used to follow response to treatment in patients with DKA. Levels greater than 0.5 mmol/L are considered abnormal, and levels of 3 mmol/L correlate with the need for treatment for DKA.
Arterial Blood Gases
In patients with DKA, arterial blood gases (ABGs) frequently show typical manifestations of metabolic acidosis, low bicarbonate, and low pH (less than 7.3).
When monitoring the response to treatment, the 2011 JBDS guideline recommends the use of venous blood rather than arterial blood in blood gas analyzers, except where respiratory problems preclude using arterial blood.
Venous pH may be used for repeat pH measurements. Brandenburg and Dire found that pH on venous blood gas in patients with DKA was 0.03 lower than pH on ABG. Because this difference is relatively reliable and not of clinical significance, there is almost no reason to perform the more painful ABG. End tidal CO2 has been reported as a way to assess acidosis as well.
Serum Electrolyte Panel
Serum potassium levels initially are high or within the reference range in patients with DKA. This is due to the extracellular shift of potassium in exchange of hydrogen, which is accumulated in acidosis, in spite of severely depleted total body potassium. This needs to be checked frequently, as values drop very rapidly with treatment. An ECG may be used to assess the cardiac effects of extremes in potassium levels.
The serum sodium level usually is low in affected patients. The osmotic effect of hyperglycemia moves extravascular water to the intravascular space. For each 100 mg/dL of glucose over 100 mg/dL, the serum sodium level is lowered by approximately 1.6 mEq/L. When glucose levels fall, the serum sodium level rises by a corresponding amount.
Additionally, serum chloride levels and phosphorus levels always are low in these patients.
Bicarbonate
Use bicarbonate levels in conjunction with the anion gap to assess the degree of acidosis that is present.
Anion Gap
In patients with diabetic ketoacidosis, the anion gap is elevated ([Na + K] – [Cl + HCO3] greater than 10 mEq/L in mild cases and greater than 12 mEq/L in moderate and severe cases).
CBC
Even in the absence of infection, the CBC shows an increased white blood cell (WBC) count in patients with diabetic ketoacidosis. High WBC counts (greater than 15 X 109/L) or marked left shift may suggest underlying infection.
Renal Function Studies
BUN frequently is increased in patients with diabetic ketoacidosis.
Osmolarity
Plasma osmolarity usually is increased (greater than 290 mOsm/L) in patients with diabetic ketoacidosis. If plasma osmolarity cannot be measured directly, it may be calculated with the following formula: plasma osmolarity = 2 (Na + K) + BUN/3 + glucose/18. Urine osmolarity also is increased in affected patients.
Patients with diabetic ketoacidosis who are in a coma typically have osmolalities greater than 330 mOsm/kg H2 O. If the osmolality is less than this in a patient who is comatose, search for another cause of obtundation.
Cultures
Urine and blood culture findings help to identify any possible infecting organisms in patients with diabetic ketoacidosis.
Amylase
Hyperamylasemia may be seen in patients with diabetic ketoacidosis, even in the absence of pancreatitis.
Phosphate, Calcium, and Magnesium
If the patient is at risk for hypophosphatemia (eg, poor nutritional status, chronic alcoholism), then the serum phosphorous level should be determined.
Chest Radiography
Chest radiography should be used to rule out pulmonary infection such as pneumonia.
MRI
An MRI is helpful in detecting early cerebral edema; it should be ordered only if altered consciousness is present.
CT Scanning
The threshold should be low for obtaining a head CT scan in children with diabetic ketoacidosis who have altered mental status, as this may be caused by cerebral edema.
Many of the changes may be seen late on head imaging and should not delay administration of hypertonic saline or mannitol in those pediatric cases where cerebral edema is suspected.
Electrocardiography
DKA may be precipitated by a cardiac event, and the physiological disturbances of DKA may cause cardiac complications. An ECG should be performed every 6 hours during the first day, unless the patient is monitored. An ECG may reveal signs of acute myocardial infarction that could be painless in patients with diabetes, particularly in those with autonomic neuropathy.
An ECG is also a rapid way to assess significant hypokalemia or hyperkalemia. T-wave changes may produce the first warning sign of disturbed serum potassium levels. Low T wave and apparent U wave always signify hypokalemia, while peaked T wave is observed in hyperkalemia.
Telemetry
Consider telemetry in patients with comorbidities (especially cardiac), known significant electrolyte abnormalities, severe dehydration, or profound acidosis.
Treatment & management
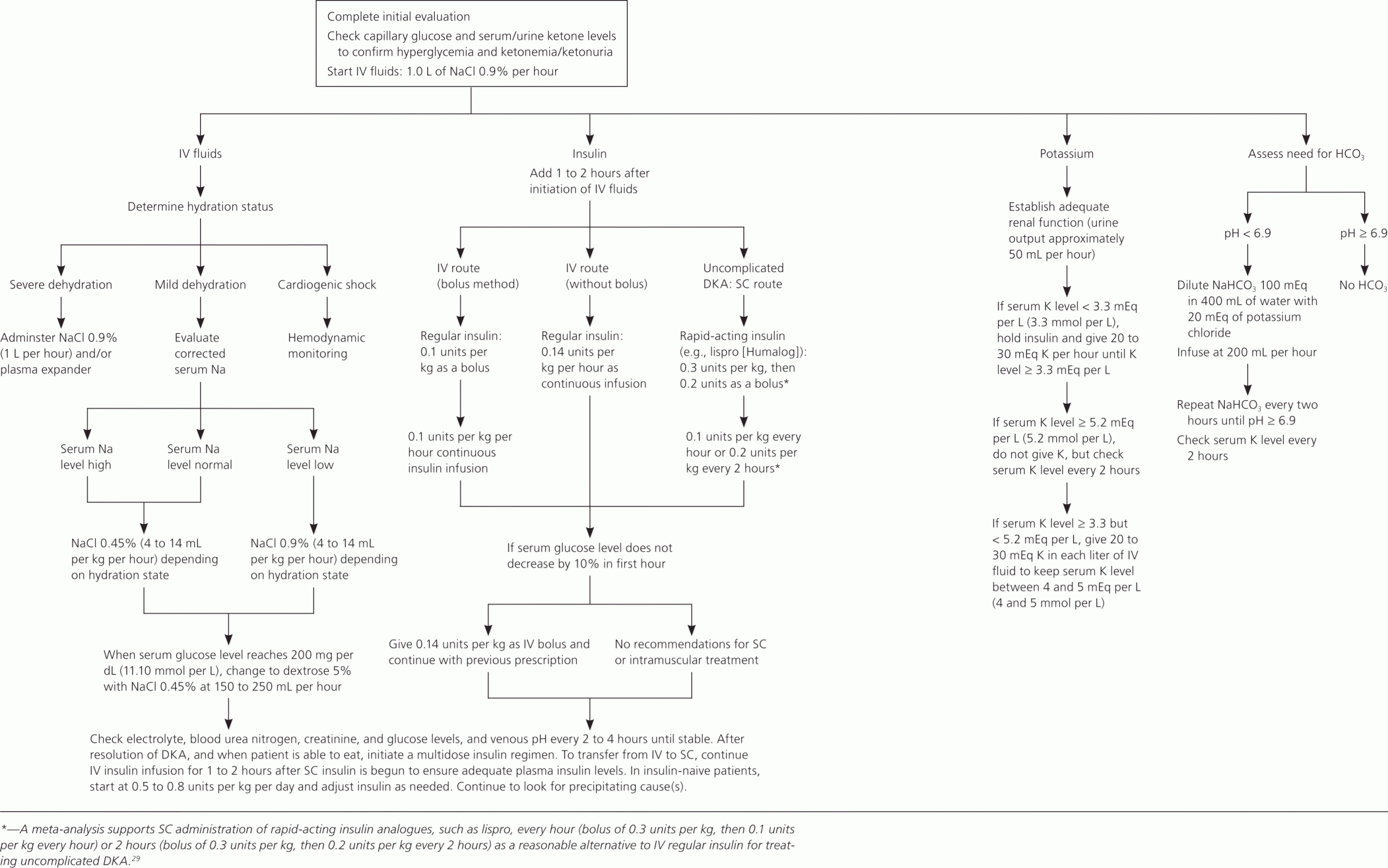
Approach Considerations
Managing diabetic ketoacidosis (DKA) in an intensive care unit during the first 24-48 hours always is advisable. When treating patients with DKA, the following points must be considered and closely monitored:
Correction of fluid loss with intravenous fluids
Correction of hyperglycemia with insulin
Correction of electrolyte disturbances, particularly potassium loss
Correction of acid-base balance
Treatment of concurrent infection, if present
It is essential to maintain extreme vigilance for any concomitant process, such as infection, cerebrovascular accident, myocardial infarction, sepsis, or deep venous thrombosis.
It is important to pay close attention to the correction of fluid and electrolyte loss during the first hour of treatment. This always should be followed by gradual correction of hyperglycemia and acidosis. Correction of fluid loss makes the clinical picture clearer and may be sufficient to correct acidosis. The presence of even mild signs of dehydration indicates that at least 3 L of fluid has already been lost.
Patients usually are not discharged from the hospital unless they have been able to switch back to their daily insulin regimen without a recurrence of ketosis. When the condition is stable, pH exceeds 7.3, and bicarbonate is greater than 18 mEq/L, the patient is allowed to eat a meal preceded by a subcutaneous (SC) dose of regular insulin.
Insulin infusion can be discontinued 30 minutes later. If the patient is still nauseated and cannot eat, dextrose infusion should be continued and regular or ultra–short-acting insulin should be administered SC every 4 hours, according to blood glucose level, while trying to maintain blood glucose values at 100-180 mg/dL.
The 2011 JBDS guideline recommends the intravenous infusion of insulin at a weight-based fixed rate until ketosis has subsided. Should blood sugar fall below 14 mmol/L (250 mg/dL), 10% glucose should be added to allow for the continuation of fixed-rate insulin infusion.
In established patients with diabetes, SC long-acting insulin (eg, insulin glargine, Detemir) should be initiated at the dose that was used prior to the manifestation of DKA. If neutral protamine Hagedorn (NPH) insulin was used previously, however, start back at the usual dose only when the patient eats well and is able to retain meals without vomiting; otherwise, the dose should be reduced to avoid hypoglycemia during its peak efficacy period.
In newly diagnosed patients with type 1 diabetes, a careful estimate of the long-acting insulin dose should be considered. Starting with smaller doses generally is recommended to avoid hypoglycemia.
A study by Lakshman et al indicated that in patients with type 1 diabetes, use of a hybrid closed-loop (HCL) system for glycemic control may help to protect against the effects of DKA. The report looked at 51 adolescents with type 1 diabetes, 17 of whom had DKA at diagnosis. All patients were placed on HCL-system therapy at diagnosis, with the investigators finding that, upon comparing those with DKA at diagnosis with those without it, the time in target glucose range, time below range, and hemoglobin A1c levels were similar at 6, 12, and 24 months. Also, after adjusting for body weight, insulin requirements were not significantly different between the two groups at 6 months. In addition, the study reported that both had similar residual C-peptide secretion. [30]
A retrospective study by Umapathysivam et al suggested that using the same treatment protocol in patients with type 2 diabetes who have SGLT2-inhibitor–associated DKA as in type 1 diabetes DKA may result in hypoglycemia in the individuals with type 2 diabetes. The investigators pointed out that although in type 1 diabetes, “DKA is driven by absolute insulin deficiency, leading to ketosis and hyperglycemia,” in SGLT2-inhibitor–associated DKA, loss of glucose through urine lowers plasma glucose, leading to a reduction in insulin secretion and stimulation of glucagon secretion, with ketosis being the result. Thus, patients with SGLT2-inhibitor–associated DKA often have only slightly elevated or normal glucose levels, with SGLT2-inhibitor–associated DKA apparently being less closely linked to glycemia than is type 1 DKA. For patients with SGLT2-inhibitor–associated DKA, according to the report, hypoglycemia may result when these individuals undergo fixed-dose insulin infusion, or they may experience inadequate insulin dosing and diminished ketone clearance, as a result of dynamic insulin infusions.
The investigators found that in the first 24 hours of treatment, study patients with SGLT2-inhibitor–associated DKA, although they had a greater median weight, received less insulin than did age-matched individuals with type 1 diabetes DKA; resolution of DKA also took longer in the former group than in the latter (median times of 36 hours vs 18 hours, respectively).
Correction of Fluid Loss
Fluid resuscitation is a critical part of treating patients with DKA. Intravenous solutions replace extravascular and intravascular fluids and electrolyte losses. They also dilute both the glucose level and the levels of circulating counterregulatory hormones. Insulin is needed to help switch from a catabolic state to an anabolic state, with uptake of glucose in tissues and the reduction of gluconeogenesis as well as free fatty acid and ketone production.
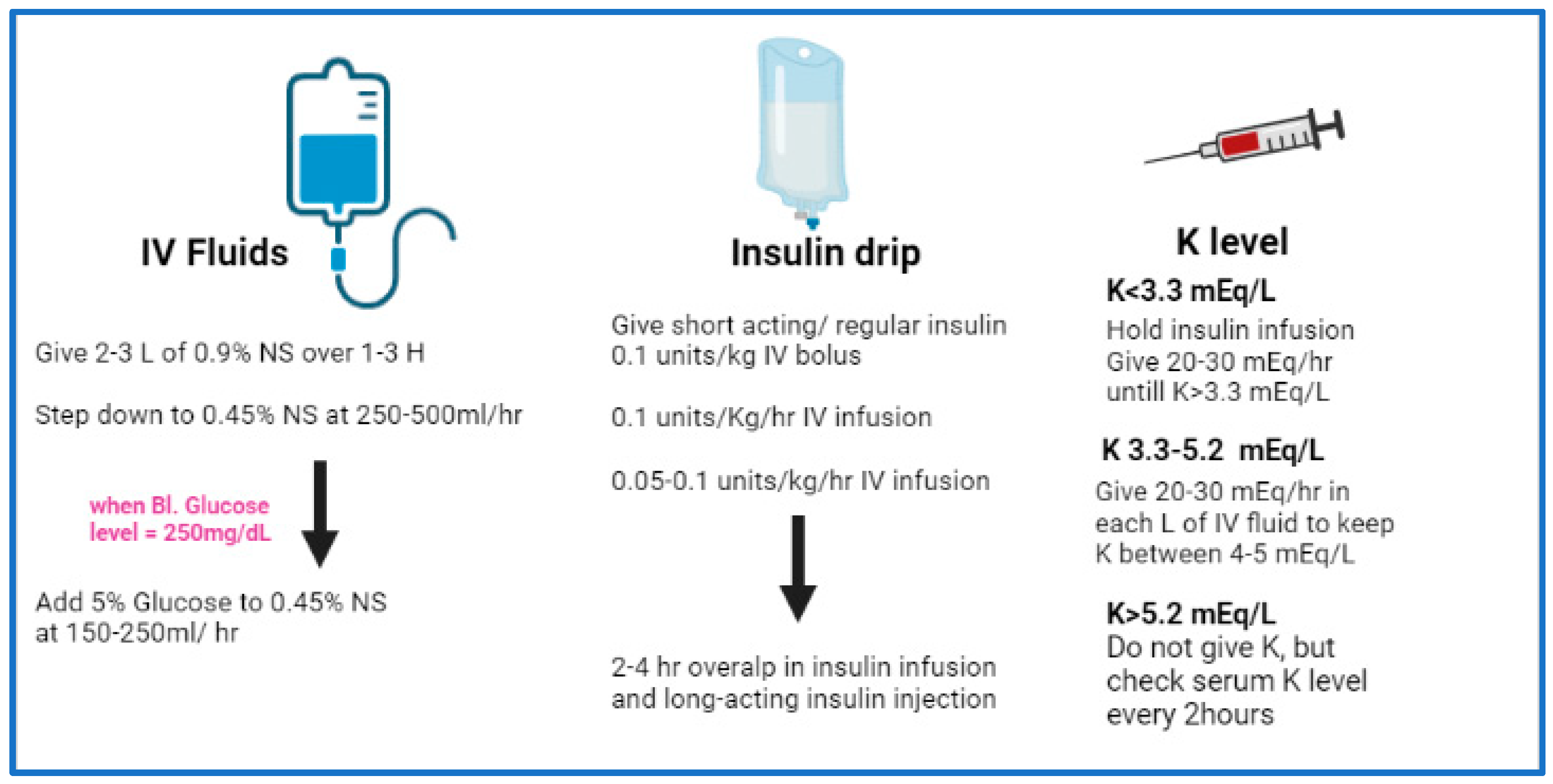
Initial correction of fluid loss is either by isotonic sodium chloride solution or by lactated Ringer solution. The recommended schedule for restoring fluids is as follows:
Administer 1-3 L during the first hour.
Administer 1 L during the second hour.
Administer 1 L during the following 2 hours
Administer 1 L every 4 hours, depending on the degree of dehydration and central venous pressure readings
When the patient becomes euvolemic, the physician may switch to half the isotonic sodium chloride solution, particularly if hypernatremia exists. Isotonic saline should be administered at a rate appropriate to maintain adequate blood pressure and pulse, urinary output, and mental status.
If a patient is severely dehydrated and significant fluid resuscitation is needed, switching to a balanced electrolyte solution (eg, Normosol-R, in which some of the chloride in isotonic saline is replaced with acetate) may help to avoid the development of a hyperchloremic acidosis.
When blood sugar decreases to less than 180 mg/dL, isotonic sodium chloride solution is replaced with 5-10% dextrose with half isotonic sodium chloride solution.
After initial stabilization with isotonic saline, switch to half-normal saline at 200-1000 mL/h (half-normal saline matches losses due to osmotic diuresis).
Insulin should be started about an hour after IV fluid replacement is started to allow for checking potassium levels and because insulin may be more dangerous and less effective before some fluid replacement has been obtained.
Although the incidence of life-threatening hypokalemia due to aggressive insulin administration is very low, there is little to no advantage in starting insulin prior to rehydration and evaluation of serum potassium levels. Initial bolus of insulin does not change overall management of DKA.
Pediatric protocols to minimize the risk of cerebral edema by reducing the rate of fluid repletion vary. The International Society for Pediatric and Adolescent Diabetes (ISPAD) Clinical Practice Consensus Guidelines suggest initial fluid repletion in pediatric patients should be 10-20 mL/kg of normal saline (0.9%) solution during the first 1-2 hours without initial bolus, and then, after 1-2 hours, insulin should be started to avoid pediatric cerebral edema.
ISPAD provides detailed fluid administration guidelines. Total volume over the first 4 hours should not exceed 40-50 mL/kg. Fluid administration is as vital in children as in adults.
Insulin Therapy
When insulin treatment is started in patients with DKA, several points must be considered. A low-dose insulin regimen has the advantage of not inducing the severe hypoglycemia or hypokalemia that may be observed with a high-dose insulin regimen.
Only short-acting insulin is used for correction of hyperglycemia. Subcutaneous absorption of insulin is reduced in DKA because of dehydration; therefore, using intravenous routes is preferable.
SC use of the fast-acting insulin analog (lispro) has been tried in pediatric DKA (0.15 U/kg q2h). The results were shown to be comparable to IV insulin, but ketosis took 6 additional hours to resolve. Such technically simplified methods may be cost-effective and may preclude admissions to intensive care units in patients with mild cases. Use of subcutaneous insulin analog (aspart) has been shown to be effective as well in adults.
The initial insulin dose is a continuous IV insulin infusion using an infusion pump, if available, at a rate of 0.1 U/kg/h. A mix of 24 units of regular insulin in 60 mL of isotonic sodium chloride solution usually is infused at a rate of 15 mL/h (6 U/h) until the blood glucose level drops to less than 180 mg/dL; the rate of infusion then decreases to 5-7.5 mL/h (2-3 U/h) until the ketoacidotic state abates.
Larger volumes of an insulin and isotonic sodium chloride solution mixture can be used, providing that the infusion dose of insulin is similar. Larger volumes may be easier in the absence of an IV infusion pump (eg, 60 U of insulin in 500 mL of isotonic sodium chloride solution at a rate of 50 mL/h).
The optimal rate of glucose decline is 100 mg/dL/h. Do not allow the blood glucose level to fall below 200 mg/dL during the first 4-5 hours of treatment. Hypoglycemia may develop rapidly with correction of ketoacidosis due to improved insulin sensitivity.
Allowing blood glucose to drop to hypoglycemic levels is a common mistake that usually results in a rebound ketosis derived by counter-regulatory hormones. Rebound ketosis necessitates a longer duration of treatment. The other hazard is that rapid correction of hyperglycemia and hyperosmolarity may shift water rapidly to the hyperosmolar intracellular space and may induce cerebral edema.
Although DKA was a common problem in patients with diabetes who were treated with continuous subcutaneous insulin infusion through insulin infusion pumps, the incidence of DKA was reduced with the introduction of pumps equipped with sensitive electronic alarm systems that alert users when the infusion catheter is blocked.
Electrolyte Correction
If the potassium level is greater than 6 mEq/L, do not administer potassium supplement. If the potassium level is 4.5-6 mEq/L, administer 10 mEq/h of potassium chloride. If the potassium level is 3-4.5 mEq/L, administer 20 mEq/h of potassium chloride.
Monitor serum potassium levels hourly, and the infusion must be stopped if the potassium level is greater than 5 mEq/L. The monitoring of serum potassium must continue even after potassium infusion is stopped in the case of (expected) recurrence of hypokalemia.
In severe hypokalemia, not starting insulin therapy is advisable unless potassium replacement is under way; this is to avert potentially serious cardiac dysrhythmia that may result from hypokalemia.
Potassium replacement should be started with initial fluid replacement if potassium levels are normal or low. Add 20-40 mEq/L of potassium chloride to each liter of fluid once the potassium level is less than 5.5 mEq/L. Potassium can be given as follows: two thirds as KCl, one third as KPO4.
Correction of Acid-Base Balance
Sodium bicarbonate only is infused if decompensated acidosis starts to threaten the patient’s life, especially when associated with either sepsis or lactic acidosis. If sodium bicarbonate is indicated, 100-150 mL of 1.4% concentration is infused initially. This may be repeated every half hour if necessary. Rapid and early correction of acidosis with sodium bicarbonate may worsen hypokalemia and cause paradoxical cellular acidosis.
Bicarbonate typically is not replaced as acidosis will improve with the above treatments alone. Administration of bicarbonate has been correlated with cerebral edema in children.
Treatment of Concurrent Infection
In the presence of infection, the administration of proper antibiotics is guided by the results of culture and sensitivity studies. Starting empiric antibiotics on suspicion of infection until culture results are available may be advisable.
Management of Treatment-Related Complications
Cerebral edema
Cerebral edema is a serious, major complication that may evolve at any time during treatment of DKA and primarily affects children. It is the leading cause of DKA mortality in children.
Be extremely cautious to avoid cerebral edema during initiation of therapy. Deterioration of the level of consciousness in spite of improved metabolic state usually indicates the occurrence of cerebral edema. MRI usually is used to confirm the diagnosis.
Cerebral edema that occurs at initiation of therapy tends to worsen during the course of treatment. Mannitol or hypertonic saline should be available if cerebral edema is suspected.
According to Wolfsdorf et al, 0.5-1 g/kg intravenous mannitol may be given over the course of 20 minutes and repeated if no response is seen in 30-120 minutes. Also, if no response to mannitol occurs, hypertonic saline (3%) may be given at 5-10 mg/kg over the course of 30 minutes.
Clinical cerebral edema is rare and carries the highest mortality rate. Although mannitol (0.25-1 g/kg IV) and dexamethasone (2-4 mg q6-12h) frequently are used in this situation, no specific medication has proven useful in such instances.
Recent research by Glaser et al indicated that cerebral edema occurs in 1% of children with DKA, with a mortality rate of 21% and neurologic sequelae in another 21% of patients. Glaser et al suggested that up to half of children with DKA have subtle brain MRI findings, particularly with respect to narrowing of the lateral ventricles.
Muir et al have identified diagnostic criteria for cerebral edema that include abnormal response to pain, decorticate and decerebrate posturing, cranial nerve palsies, abnormal central nervous system respiratory patterns, fluctuating level of consciousness, sustained heart rate deceleration, incontinence, and more nonspecific criteria such as vomiting, headache, lethargy, and elevated diastolic blood pressure.
Cerebral edema begins with mental status changes and is believed to be due partially to idiogenic osmoles, which have stabilized brain cells from shrinking while the diabetic ketoacidosis was developing.
The risk of cerebral edema is related to the severity and duration of DKA. It is often associated with ongoing hyponatremia. Cerebral edema is correlated with the administration of bicarbonate. Concerns about the role of overaggressive or overly hypotonic fluid resuscitation as a cause of the edema that have been raised in the past correlate more closely with disease severity than with rapid administration of fluids.
Cardiac dysrhythmia
Cardiac dysrhythmia may occur secondary to severe hypokalemia and/or acidosis either initially or as a result of therapy in patients with DKA. Usually, correction of the cause is sufficient to treat cardiac dysrhythmia, but if it persists, consultation with a cardiologist is mandatory. Performing cardiac monitoring on patients with DKA during correction of electrolytes always is advisable.
Pulmonary edema
Pulmonary edema may occur for the same reasons as cerebral edema in patients with diabetic ketoacidosis. Be cautious of possible overcorrection of fluid loss, though it occurs only rarely.
Although initial aggressive fluid replacement is necessary in all patients, particular care must be taken in those with comorbidities such as renal failure or congestive heart failure. Diuretics and oxygen therapy often suffice for the management of pulmonary edema.
Myocardial injury
Nonspecific myocardial injury may occur in severe DKA, which is associated with minute elevations of myocardial biomarkers (troponin T and CK-MB) and initial ECG changes compatible with myocardial infarction (MI).
Acidosis and very high levels of free fatty acids could cause membrane instability and biomarker leakage. Coronary arteriography usually is normal, and patients tend to recover fully without further evidence of ischemic heart disease. Regardless of the pathogenesis, the presence of minute biomarker elevations and ECG changes do not necessarily signify MI in DKA.
Diabetic retinopathy
Microvascular changes consistent with diabetic retinopathy have been reported prior to and after treatment of diabetic ketoacidosis; the blood-retinal barrier does not experience the same degree of perturbation as the blood-brain barrier does, however.
Hypoglycemia
In patients with diabetic ketoacidosis, hypoglycemia may result from inadequate monitoring of glucose levels during insulin therapy. Insulin sensitivity improves after clearance of ketones.
Hypokalemia
Hypokalemia is a complication that is precipitated by failing to rapidly address the total body potassium deficit brought out by rehydration and insulin treatment, which not only reduces acidosis but directly facilitates potassium reentry into the cell.
Consultations
An endocrinologist also may be consulted to assist with management after the patient has been stabilized adequately.
Any mental status change in pediatric patients suggests the possibility of cerebral edema, and when this occurs, a pediatric endocrinologist or pediatric intensivist should be consulted as soon as possible. Psychological counseling of young children and adolescents usually is helpful.
Long-Term Monitoring
Frequent blood glucose monitoring at home makes DKA less likely, as this allows them to promptly search for possible reasons for unexpectedly high blood sugar values before the condition progresses to DKA.
In a study of 127 patients with DKA who were admitted to a pediatric intensive care unit, Bradley and Tobias concluded that multiple weaknesses existed in the prehospital care of these patients. These included lack of appropriate laboratory evaluation, excessive insulin dosing (both bolus doses and infusion rates), lack of fluid resuscitation, use of inappropriate fluids for resuscitation, and the use of sodium bicarbonate.
Medication Summary
Regular and analog human insulins are used for correction of hyperglycemia, unless bovine or pork insulin is the only available insulin. Clinical considerations in treating diabetic ketoacidosis (DKA) include the following:
Only short-acting insulin is used for correction of hyperglycemia in DKA.
The optimal rate of glucose decline is 100 mg/dL/h.
The blood glucose level should not be allowed to fall lower than 200 mg/dL during the first 4-5 hours of treatment.
Avoid induction of hypoglycemia because it may develop rapidly during correction of ketoacidosis and may not provide sufficient warning time.
Treatment of ketoacidosis should aim to correct dehydration, reverse the acidosis and ketosis, reduce plasma glucose concentration to normal, replenish electrolyte and volume losses, and identify the underlying cause.
According to the 2011 JBDS DKA guideline, patients who are already taking long-acting insulin analogues such as glargine or detemir should be maintained at their usual doses.
Rapid-acting insulins
Class Summary
Rapid-acting insulins have a rapid onset and short duration of action and are associated with less hypoglycemia than regular insulin.
Insulin aspart (NovoLog)
Insulin aspart has an onset of action of 5-15 minutes. The peak effect occurs within 30-90 minutes, and its usual duration of action is 4 hours.
Insulin glulisine (Apidra)
Insulin glulisine has an onset of action of 5-15 minutes. The peak effect occurs within 30-90 minutes, and its usual duration of action is 4 hours.
Insulin lispro (Humalog)
Insulin lispro has an onset of action of 5-15 minutes, and its usual duration of action is 4 hours.
Short-acting insulins
Class Summary
Insulin suppresses hepatic glucose output and enhances glucose uptake by peripheral tissues. Insulin also suppresses ketogenesis and lipolysis, stimulates proper use of glucose by the cells, and reduces blood sugar levels. Only short-acting insulin is used for correction of hyperglycemia.
Regular insulin (Humulin R, Novolin R)
Regular insulin has an onset of action of 0.5-1 hours. Its peak effect occurs within 2-4 hours, and its usual duration of action is 4-6 hours.
Electrolyte Supplement
Class Summary
Serum potassium levels initially are high or within the reference range in patients with DKA. This needs to be checked frequently, as values drop very rapidly with treatment. Supplements such as potassium chloride work to correct such electrolyte imbalances.
Potassium chloride (Klor-Con, K-Dur, Kaon Cl)
Potassium deficits are high in patients with diabetic ketoacidosis, even with paradoxically high K+ due to acidotic state, which shifts H+ into cells and K+ out of cells into blood. Repletion with potassium phosphate often thought unnecessary, although some recommend giving potassium phosphate to replete both of these electrolytes. Potassium replacement should be started with initial fluid replacement if potassium levels are normal or low. Monitor the potassium level every 1-2 hours initially.
Alkalinizing Agents
Class Summary
These agents may be used as a temporizing measure in very severe acidosis and in patients who become hemodynamically unstable because of the acidosis.
Sodium bicarbonate (Neut)
Sodium bicarbonate is only infused if decompensated acidosis starts to threaten the patient’s life, especially when associated with either sepsis or lactic acidosis. If sodium bicarbonate is indicated, 100-150 mL of 1.4% concentration is infused initially. This may be repeated every half hour if necessary. Rapid and early correction of acidosis with sodium bicarbonate may worsen hypokalemia and cause paradoxical cellular acidosis.