Normocytic Anaemia
This refers to anaemia with normal-sized RBCs (MCV = 80-100). It is mainly due to 2 types of disease:
– Increased peripheral destruction –> haemolytic anaemias
– Underproduction by the bone marrow –> aplastic anaemia, chronic kidney disease
– To distinguish between peripheral destruction and underproduction by the bone marrow, you look at the reticulocyte count
Reticulocyte Count
Intravascular Haemolysis
These conditions involve destruction of RBCs within blood vessels and lead to following signs:
– Haemoglobinaemia –> raised free plasma Hb in serum
– Haemoglobinuria –> this causes tea-coloured urine
– Decreased Plasma haptoglobin –> This mops up free plasm Hb, and is then removed by the liver
– Haemosiderinuria –> the occurs when the haptoglobin-binding capacity is exceeded causing Hb to be filtered and freely absorbed by tubule cells which store it as hemosiderin, and then shed in urine
Paroxysmal Nocturnal Haemoglobinuria (PNH)
This is an acquired stem cell disorder resulting in absent GPI, making cells susceptible to complement lysis
– GPI usually anchors decay accelerating factor (DAF) to cell which inhibits complement
– The lack of DAF means complement lyses cells releasing bilirubin, LDH within blood vessels
– Leads to intravascular haemolysis occasionally, especially in night during sleep
– This is because mild respiratory acidosis in sleep gives shallow breathing which activates complement
– Lack of CD59 on platelet membranes also causes platelet aggregation leading to thrombosis
Symptoms:
– Haemoglobinuria (tea-coloured urine in the morning) + Anaemia symptoms
– Thrombosis –> hepatic veins (Budd-Chiari syndrome), cerebral veins
– Can lead to aplastic anaemia and acute myeloid leukhaemia
Diagnosis:
– Flow cytometry –> detects lack of CD55 and CD59 on blood cells
Management:
– Anticoagulation to reduce chances of thrombosis + Blood product replacement for anaemia
– Monoclonal anticomplement antibodies e.g. Eculizumab
– Bone marrow transplantation
Autoimmune haemolytic anaemia (AIHA)
This is antibody-mediated destruction of RBCs, which is usually due to IgG or IgM antibodies.
– It is diagnosed with the Coombs test:
Coombs Test
IgG:
This coats RBCs allowing them to be removed by the spleen (extravascular haemolysis)
– The IgG antibody usually binds RBC in the warm temperature of the central body.
Causes:
SLE (autoimmune antibodies) + Lymphoma and drugs (penicillin + methyldopa)
Management:
Stop the drug, steroids/immunosuppression and if necessary splenectomy
IgM:
This coats RBCs which leads to complement dependent lysis (intravascular disease)
– The IgM antibody usually causes haemolysis in cold temperature i.e. in the extremities -Causes a chronic anaemia that is made worse by the cold
Causes:
Infective causes (EBV) and lymphoma
Management:
Keep warm
Extravascular Haemolysis
These conditions cause RBC destruction by the reticuloendothelial system (macrophages in spleen, liver)
– The haemoglobin is broken down into amino acids (protein chains) and unconjugated bilirubin (haem), which collects in the blood giving rise to jaundice.
General Symptoms:
– Splenomegaly due to increased work by organs
– Unconjugated bilirubin causes jaundice + bilirubin gallstones
– Bone marrow tries to compensate by increasing the reticulocyte count > 3%.
Glucose-6-phosphate dehydrogenase deficiency
This is an X-linked recessive disorder
– It is the most common red blood cell enzyme defect which gives a deficiency of the enzyme G6PD, which makes reduced glutathione
– Without this, RBCs are susceptible to oxidative stress due to a variety of different causes such as drugs, illness and fava beans
– The oxidative stress causes Hb to precipitate as Heinz bodies which are removed by macrophages making bite cells
– Higher frequency in Africans and Mediterranean, due to malaria protection
Causes:
– Primaquine, Ciprofloxacin, Sulphur-drugs (sulphasalazine, sulphonamides)
– Infection can also precipitate haemolysis
Symptoms:
Presents often with jaundice in newborn babies within the first 24 hours
– Gives acute attacks with symptoms of both intra and extravascular haemolysis
– Intravascular –> jaundice, anaemia
– Extravascular –> splenomegaly, gallstones
Tests:
Blood film shows bite and blister cells, with Heinz Bodies
– Definitive diagnosis by enzyme assay (taken 8 weeks after attack, otherwise not accurate)
Pyruvate Kinase deficiency
This is where decreased pyruvate kinase gives ATP depletion meaning RBCs are unable to survive.
– Majority of patients are detected at birth whilst some only present with symptoms during stress
Symptoms:
Gives neonatal jaundice, followed by later haemolysis with splenomegaly
Diagnosis:
Enzyme assay
Management:
Often not required, but blood transfusions if severe.
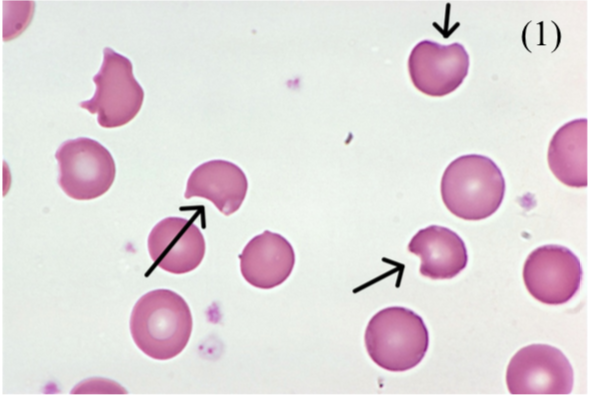
Hereditary Spherocytosis
This is an autosomal dominant defect of RBC cytoskeleton, giving inflexible spherical RBCs
– They are unable to move through spleen sinusoids so get consumed by macrophages
– MCHC (mean corpuscular hemoglobin concentration) is raised
Symptoms:
Failure to thrive + Anaemia with splenomegaly + Jaundice
– Can get an aplastic crisis with a parvovirus infection
Diagnosis:
– Originally osmotic fragility test (increased fragility in hypotonic solution)
– Now we use the cryohaemolysis test and EMA binding
Management:
Splenectomy (resolves anaemia) + Folate replacement
Sickle Cell Anaemia
This is an autosomal recessive mutation in the b-chain where a glutamate is replaced with valine.
– This leads to production of HbS rather than HbA, but HbA2 and HbF are still produced
– Homozygotes have HBSS (sickle cell anaemia) whereas heterozygotes have HbAS (sickle-cell trait)
i) Sickle-cell trait
This is generally asymptomatic with no anaemia and protects against P. Falciparum
– RBCs do not sickle except in kidney in hypoxia e.g. altitude or anaesthesia, which can lead to microscopic haematuria and kidney injury
– Therefore, those of African descent given a pre-operation (anaesthesia) sickle-cell test
ii) Sickle Cell Anaemia
HbS polymerizes in low oxygen, causing RBCs to sickle and get stuck
– HbF at birth is protective for few months and hydroxyurea treatment increases HbF levels.
-However, later you get increase extravascular haemolysis + intravascular haemolysis
Symptoms:
It is characterized by periods of good health with intervening crises:
Sequestration crisis:
– Blood becomes trapped in the spleen and liver (giving organomegaly),
– Leads to severe anaemia and shock, requiring urgent transfusion
Aplastic crisis:
– Parvovirus infection leading to sudden fall in Haemoglobin
Acute chest syndrome:
– Occlusion of pulmonary microcirculation gives chest pain, SOB, bleeding.
– Most common cause of death in adult patients.
Thrombotic (Vaso-occlusive) crises:
– RBCs occlude in blood vessels leading to infarction of organs
– Gives Hand-foot syndrome –> swollen hands/feet due to occlusion, common in infants
– Autosplenectomy –> infarction gives shrunken, fibrotic spleen increasing infection risk
– Pain –> ischaemia of bone marrow gives unbearable pain
Tests:
– Diagnosed by Hb electrophoresis
– Blood Film –> shows target and sickle cells
– Sickle solubility test –> Does not distinguish hetero- and homozygotes
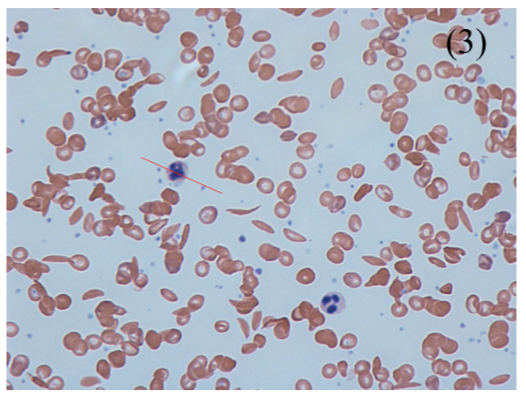
Management:
– Refer to haematology
– In infant, hydroxyurea increases levels of HbF
– Prophylactic antibiotics in splenic infarction, but control Hb with transfusions
Underproduction Conditions
These conditions are marked by decreased RBC production, characterised by a low reticulocyte count.
– In acute setting due to blood loss + onset of iron deficiency and anaemia of chronic disease
– In chronic due to 2 main conditions: Renal failure (decreased production of EPO) + Aplastic anaemia
Aplastic anaemia
This is a stem cell disorder in which the bone marrow stops making cells, leading to pancytopenia
Causes:
– Idiopathic + radiation + cytotoxic drugs + viruses (parvovirus)
– (Congenital) Fanconi anaemia –> autosomal recessive aplastic anaemia + short + microcephaly
Symptoms:
– Bone marrow failure –> Anaemia + susceptibility to infections + bleeding
– A minority of patients develop paroxysmal nocturnal hemoglobinuria or myelodysplasia
Tests:
Bone marrow biopsy is diagnostic –> Reveals an empty, fatty marrow
Management:
– Stop causative drugs + supportive care (transfusion and marrow stimulating factors)
– In young, treatment of choice is bone marrow transplant
– Immunosuppression with ciclosporin as some cases might be due to autoimmunity
Parvovirus B19
This is a respiratory DNA virus which usually affects children and has several different presentations
– Symptoms start 6 days after exposure and last about week
– Most common presentation is erythema infectiosum (fifth disease / “slapped-cheek syndrome”)
Symptoms:
– By the time you develop symptoms, you are no longer contagious (so do not avoid school)
– Very mild fever, malaise and a mild arthritis
– Rose red rash that makes cheeks appear bright red (like “slapped cheeks”) – does not involve hands/feet
N.B. This virus also infects progenitor red cells and temporary stops erythropoiesis for about a week
– Usually asymptomatic, but can lead to a significant anaemia if the patient has a preexisting disease (e.g. sickle cell anaemia or hereditary spherocytosis)
– If reticulocytes are very low (or not raised) suspect an aplastic crisis (shows bone marrow is not capable of compensatory reticulocytosis)

