Leukaemias
This is a neoplastic proliferation of WBC cell types which do not form a mass, divided into acute and chronic:
Leukhaemias will usually be diagnosed in the follow pathway:
– FBC –> shows raised WBC count
– Blood smear –> shows presence of tumour cells in the blood
– Bone marrow biopsy with immunophenotyping is diagnostic –> shows >20% blasts with cell markers
– CT staging scan allows for staging and to look for organ involvement
NICE Referral Guidelines
Acute Leukaemia
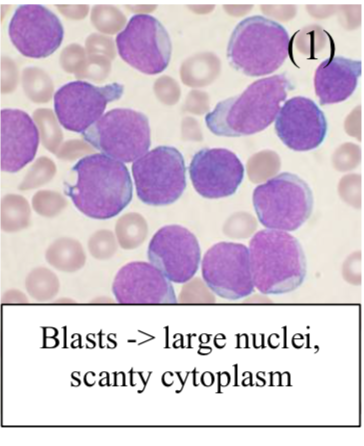
This is the neoplastic proliferation of (WBC precursors “blasts”), defined as >20% blasts in bone marrow
– Increased blasts disrupt normal hematopoiesis, resulting in a quick-onset presentation with anaemia (fatigue), thrombocytopenia (bleeding) and neutropenia (infection).
Acute Lymphoblastic Leukaemia (ALL)
This is a neoplastic proliferation of immature lymphoblasts in the bone marrow, which leads to bone marrow failure and tissue infiltration. It is the most common malignancy of childhood (rarer in adults)
– The cancer cells have a marker, which is the enzyme TdT and are divided into B or T cell lymphoblasts
– B cell lymphoblasts display cell surface markers CD19+ but lack CD20+ and the B cell receptor
– T cell lymphoblasts display CD3+ (a marker of T cells), but have not yet developed CD4+/CD8+
Symptoms:
– Bone marrow failure –> Anaemia (low Hb), infection (low WCC) and bleeding (low platelets)
– Fever –> due to infection after bone marrow failure or the B cells themselves
– Tissue infiltration –> Hepato/splenomegaly + Testicular swelling + CNS involvement
– Bone pain –> secondary to bone marrow infiltration
Tests:
Bone marrow biopsy shows blast cells
– CXR/CT + Lumbar puncture –> looks for external organ/CNS infiltration
Management:
– Chemotherapy –> used to induce remission and then maintain
– Bone marrow transplant can be curative
Prognosis:
Cure rates are up to 90% for children based on cytogenetic abnormalities of the cancer cells
Acute Myeloid Leukaemia (AML)
This is a proliferation of immature myeloid cells in the bone marrow
– It is the commonest acute leukaemia of adults, with increasing incidence the older you get
– It is subclassified into different types based on cytogenetics, lineage and surface markers:
Most common type is Acute promyelocytic leukaemia:
This involves translocation of Chr 17 to 15
– Gives fusion of PML and RAR-alpha gene, and presents at a much younger age than normal
– It is managed with all-trans-retinoic acid which binds to the altered receptor allowing blasts to mature
Causes:
– Can arise from chronic myeloid leukaemia, or prior exposure to radiotherapy or alkylating agents
Symptoms:
– Marrow failure –> Anaemia + Bleeding + Infection
– Infiltration of cells –> Hepatomegaly, splenomegaly + Bone Pain + Gum hypertrophy
– Leukaemia cutis – purple patches in the skin due to blast infiltration into the dermis
Tests:
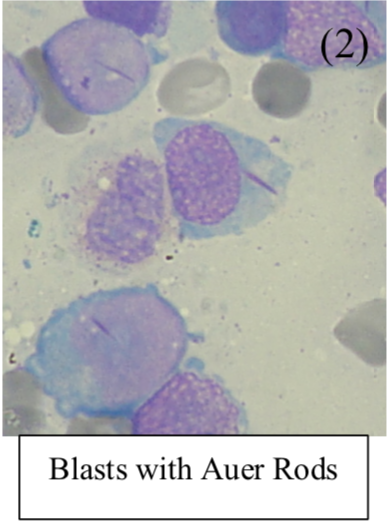
– Bone marrow biopsy shows blast cells with Auer rods which also stain positive for myeloperoxidase
Management:
– Chemotherapy with Daunorubicin + Cytarabine (cytosine drug stops DNA synthesis)
– Bone marrow transplant
Chronic Leukaemia
This is a neoplastic proliferation of mature circulating lymphocytes, characterized by a high WBC count
– These usually are insidious in onset and seen in older adults.
Chronic Lymphocytic Leukaemia (CLL)
A neoplastic proliferation of B cells which co-express CD5 and CD20, most common leukemia in adults.
– The defining feature is an accumulation of non-functional B cells leading to complications
– Unlike the lymphoblasts, these cells are more mature, so they display different receptor
– B cells at this maturity display CD19+ and CD20+, as well as the B cell receptor
– T cells at this maturity display CD3+ as well as either CD4+ or CD8+
Symptoms:
Often asymptomatic, presenting as an incidental finding on a FBC (4)
– B cell driven –> Anorexia, weight loss, night sweats
– Patients can be anaemic or infection prone
– Involvement of lymph nodes leads to generalize lymphadenopathy (splenomegaly)
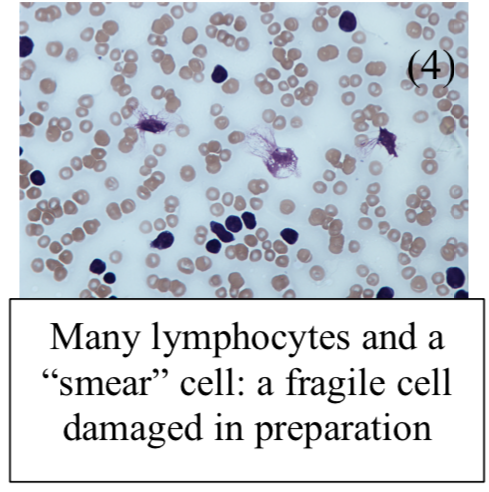
Blood film:
Shows smear/smudge cells
Complications:
– Autoimmune hemolytic anaemia (IgG mediated)
– Bone marrow failure –> leads to anaemia and hepatosplenomegaly
– Hypogammaglobinemia (low IgG) –> this leads to infection, most common cause of death in CLL
A dangerous complication is transformation of B-cell lymphoma (Richter’s syndrome):
– Occurs when leukaemia cells enter lymph nodes and changes into a high-grade non-Hodgkin’s lymphoma
– Patients become ill very suddenly with weight loss, nausea, lymph node swelling and fevers
Management:
– In those without or minimal symptoms, usually just watchful waiting
– FCR: Fludarabine (purine analog stops DNA synthesis) + Cyclophosphamide + Rituximab
Chronic Myeloid Leukaemia (CML)
This is a neoplastic proliferation of mature myeloid cells, such as basophils.
– It occurs most often in elderly people around the age of 70 years and is rare in childhood,
– Due to 9 –> 22 chromosome translocation (Philadelphia Chromosome) which makes a BCR-ABL fusion protein (tyrosine kinase) with excess activity of the tyrosine kinase.
– Starts off chronic, but then gives accelerated phase with more symptoms and then blast transformation into an acute leukhaemia
Symptoms:
Usually insidious onset –> weight loss, tiredness, fever, sweats
– Gives anaemia and lethargy + increase in granulocytes + thrombocytosis
– Massive splenomegaly
– Progresses to acute lymphoblastic or acute myeloid leukaemia
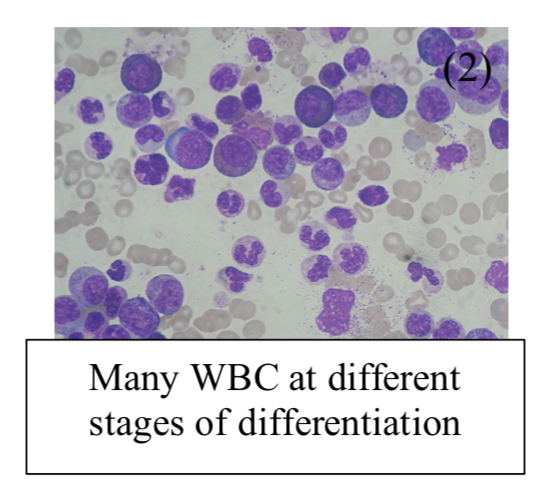
Blood film:
– Shows many WBC at different stages of differentiation
– Increase in band cell number –> myeloid progenitor cells in the bloodstream
Management:
– 1st line is BCR-ABL inhibitor = Imatinib (small molecule inhibitor against the tyrosine kinase)
– Bone marrow transplant (usually only in younger patients)
Adult T Cell Leukaemia
This is a neoplastic proliferation of mature CD4+ T cells, associated with the virus HTLV-1
– It is most commonly seen in the Caribbean and Japan.
Symptoms:
– Rash (skin infiltration)
– Generalised lymphadenopathy
– Hepatosplenomegaly
– Hypercalcemia due to lytic bone lesions

