Introduction
Acute myeloid leukaemia occurs due to the malignant transformation and proliferation of myeloid progenitor cells.
Leukaemia
Leukaemia refers to a group of malignancies that arise in the bone marrow. They are relatively rare but together are the 12th most common cancer in the UK, responsible for around 4,700 deaths a year.
There are four main types:
- Acute myeloid leukaemia (AML)
- Acute lymphoblastic leukaemia (ALL)
- Chronic myeloid leukaemia (CML)
- Chronic lymphocytic leukaemia (CLL)
Presentation, prognosis and management all depend on the type and subtype of leukaemia.
Acute myeloid leukaemia
In AML proliferation of abnormal myeloid progenitor cells is seen. It refers to a heterogeneous group of conditions characterised by different genetic mutations.
Chemotherapy forms the mainstay of management in the form of induction and consolidation regimens with stem cell transplantation used in select patients.
Epidemiology
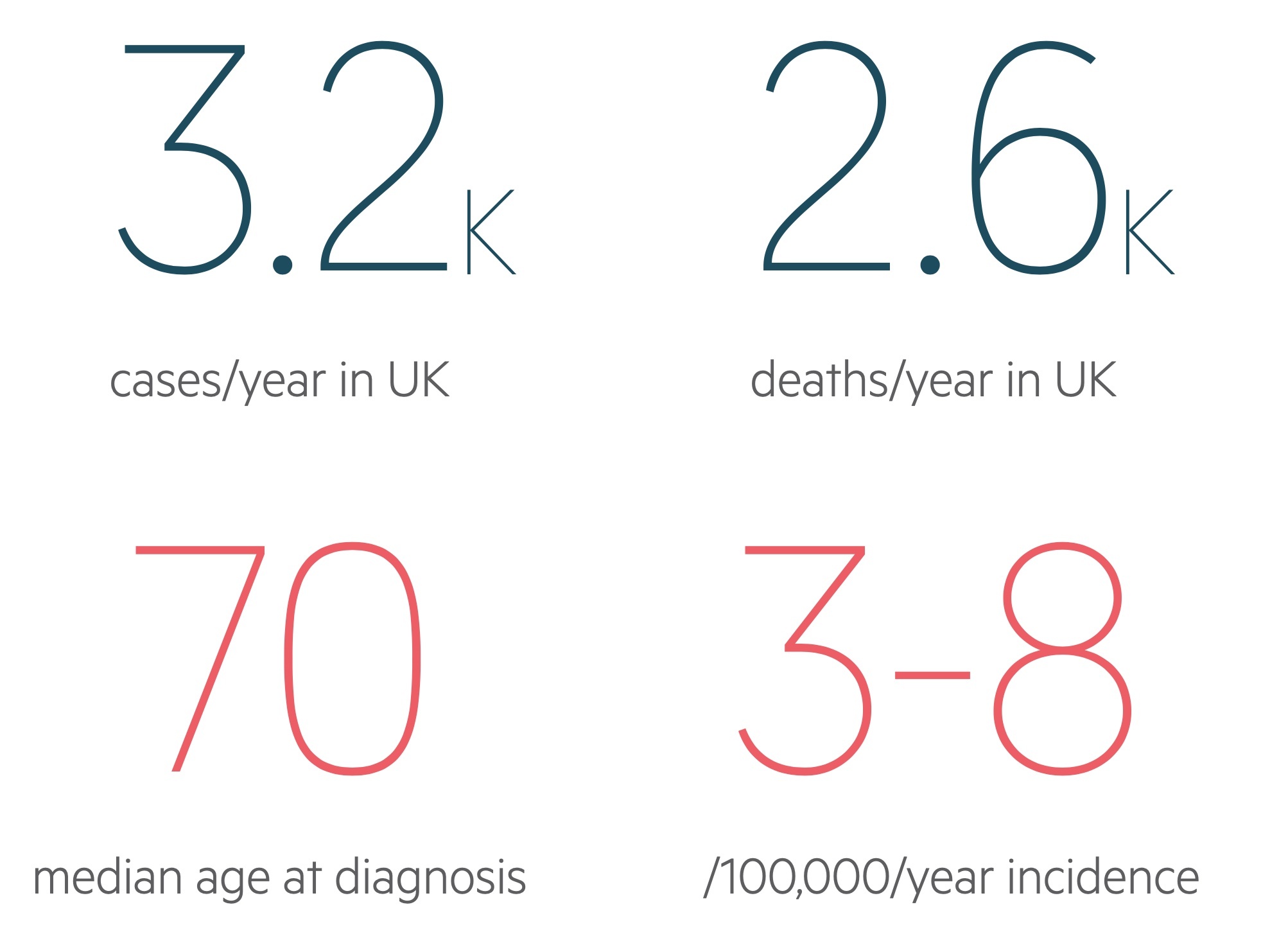
There are approximately 3,200 cases of AML in the UK each year.
Overall it is rare accounting for less than 1% of new cancers in the UK each year. It is somewhat more common in men in the UK affecting around 1,800 males compared to 1,400 females.
Incidence increases with age, becoming much more common in the over-60s with a median age at diagnosis of around 70.
Aetiology
The aetiology of AML is not completely understood.
The majority of patients present without clearly identifiable causative factors. However in some cases factors such as those described below are identified:
- Myelodysplastic syndrome: MDS refers to a heterogenous group of blood disorders affecting haematopoiesis. These patients are at increased risk of developing AML, in particular those with high risk features (e.g. excessive blasts).
- Congenital disorders: AML is seen more commonly in a number of congenital conditions including Down’s syndrome, congenital neutropaenia and Fanconi anaemia.
- Radiation exposure: Is known to result in genetic damage with the rate of leukaemia increased in the survivors of the atomic blasts in Hiroshima and Nagasaki.
- Previous administration of chemotherapy: Previous chemotherapy regimens, in particular alkylating agents and topoisomerase-II inhibitors, are associated with increased rates of AML.
- Toxins: Exposure to certain toxins (e.g. benzene and organochlorine insecticides) have been shown to increase the risk of AML.
Pathogenesis
AML occurs due to genetic changes in myeloid progenitor cells leading to a proliferating line of immature cells not responsive to normal control mechanisms.
There are complex group of genetic changes that can lead to AML including point mutations, translocations and gene silencing.
The abnormal proliferating cells result in reduced production of normal haematopoietic cell lines with abnormal cells accumulating in the bone marrow. This may be followed by spread and proliferation into other tissues.
Clinical features
AML often presents with signs of marrow failure or tissue infiltration.
Marrow failure
- Anaemia:
- Fatigue
- Breathlessness
- Angina
- Neutropenia:
- Recurrent infections
- Thrombocytopenia:
- Petechiae
- Nose bleeds
- Bruising
Tissue infiltration
The involvement and proliferation of lymphoid progenitors in body tissues is often clinically apparent at diagnosis:
- Lymphadenopathy
- Hepatosplenomegaly
- Bone pain
- Gum hypertrophy
- Violaceous skin deposits
- Testicular enlargement
Hepatomegaly and splenomegaly may cause symptoms such as early satiety or reduced appetite.
Leucostasis
May occur due to large numbers of white cells entering the bloods stream. Organ dysfunction may result due to impairment of flow through small blood vessels. Features include:
- Altered mental state
- Headache
- Breathlessness
- Visual changes
Investigations & diagnosis
Bone marrow aspirate and biopsy is required for formal diagnosis of AML.
Bloods
A normocytic normochromic anaemia is common as is thrombocytopenia and reduced reticulocyte counts. The majority will have a raised white cell count, though leucopenia is commonly encountered. Circulating myeloblasts are seen in around 95% of patients.
Electrolyte abnormalities such as hyperuricaemia and hyperkalaemia may be seen in tumour lysis syndrome – though this most commonly occurs after the initiation of treatment.
Clotting screen is important to establish a baseline and look for biochemical evidence of disseminated intravascular coagulation (DIC) a possible complication of AML.
LDH a non-specific marker of increased cell turnover may be raised in leukaemia.
- FBC
- Renal function
- LFTs
- Clotting screen
- DDIMER
- Bone profile & Mg
- Uric acid
- LDH
- Blood borne virus screen
Blood smear should be completed in all patients. Auer rods – azurophilic structures seen in myeloid blasts – is the classical finding on peripheral blood film. They are also seen in myelodysplastic syndrome.
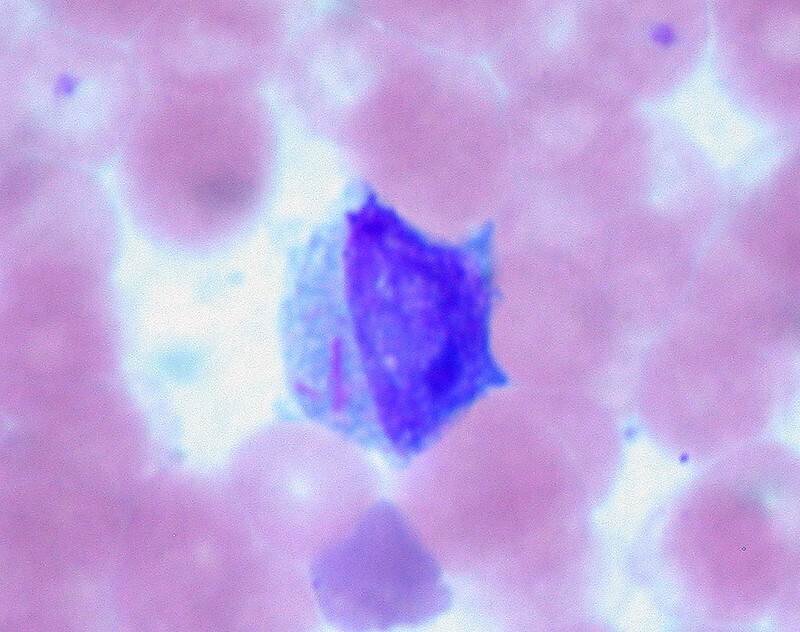
Auer Rods in a patient with AML (two pink lines on left side of cell)
Image courtesy of Ed Uthman
Bone marrow aspirate and biopsy
A sample of bone marrow allows for a definitive diagnosis. AML is defined based on bone marrow findings with a myeloid blast count of > 20% (of 500 bone marrow cells).
Aspirate and biopsy samples are used for cytogenetics, immunophenotyping and flow cytometry.
Lumbar puncture
Should be organised if there is concern of CNS involvement.
Management
As with all the haematological malignancies management is extremely complex.
Here we will give a general overview of the key points for the management of non-acute promyelocytic leukaemia AML.
Initial management
Patients should be transferred to and managed in specialist centres. Where appropriate they are enrolled in clinical trials.
- Education and support: Patients (and their families) should be offered education and support for their diagnosis, normally co-ordinated by a specialist nurse.
- Supportive care: Patients should be monitored for infections and coagulopathy and treated accordingly.
- Cytoreduction: Should be considered in patients with signs of leukostasis and WBC > 100 × 109/L. This is typically achieved with hydroxycarbamide.
- CNS involvement: Those with CNS involvement will normally be started on intrathecal chemotherapy (cytarabine).
- Tumour lysis syndrome: Should be anticipated, prophylaxis should be given where indicated and appropriate medical services available (HDU/ITU) to offer organ support if significant TLS occurs.
Chemotherapy and haematopoietic stem cell transplantation
Treatment is set up in cycles and organised into induction and consolidation (and occasionally maintenance) stages.
Induction takes the form of intensive chemotherapy and is followed by further stages of consolidation therapy. A simplified example regime is given below:
- Induction: 7+3 GO – An induction regime consisting of cytarabine, daunorubicin and gemtuzumab ozogamicin (GO).
- Consolidation: IDAC +/- GO – A consolidation regime consisting of intermediate-dose cytarabine (IDAC) +/- gemtuzumab ozogamicin.
AML is the most common indication for allogenic haematopoietic stem cell transplantation (allo HCT). In select patients allogenic haematopoietic stem cell transplantation forms part of the consolidation therapy, in others it may be used after treatment failure (i.e. molecular progression / relapse after chemotherapy). Patients are given myeloablative conditioning regimes (+/- total body irradiation) aimed at eliminating disease and allowing for a graft (the allo HCT) versus leukaemia reaction.
It is beyond the scope of these notes to give a detailed overview of each possible regimen but for those interested check out the ESMO guidelines.
Prognosis
Advancing age and poor performance status are strongly associated with a poorer prognosis.
Survival is highly variable with younger, fitter patients having better outcomes. It is known that ‘secondary leukaemia’ – those following a preceding haematological disorder (e.g. MDS) – have poorer outcomes.
Adverse prognostic factors include:
- Age (> 60)
- Poor performance status
- Multiple significant co-morbidities
- Previous haematological disorders / dysplasia
- Previous exposure to chemo/radio-therapy
- Certain disease subtypes
Cancer Research UK does not have UK wide figures for AML. However figures from 2010 show around 20% survive for 5-years or more after their diagnosis. The five-year survival is highly dependent on age:
- < 14: 65%
- 15-24: 60%
- 25-64: 40%
- > 65: 5%

