Overview
Primary aldosteronism is a condition caused by an excess of the adrenal hormone aldosterone independent of the renin-angiotensin-aldosterone axis.
The hallmarks of disease are hypertension and hypokalaemia – though hypokalaemia is often absent. Primary aldosteronism is increasingly being recognised as a common and under-diagnosed cause of secondary hypertension. Today prevalence is thought to be between 5-15% of patients with hypertension.
Disease is caused by one of a number of subtypes:
- Bilateral idiopathic hyperaldosternism (60-70%)
- Aldosterone-producing adenoma (30-40%)
- Unilateral hyperplasia (approx 3%)
- Other (familial hyperaldosteronism, adrenal carcinoma)
Treatment depends on the underlying cause and whether it is unilateral or bilateral. It may involve surgery (adrenalectomy) or aldosterone antagonists (e.g. spironolactone).
The adrenal gland
The adrenal gland is divided into two functionally distinct components, an outer cortex and an inner medulla.
Also termed the ‘suprarenal’ gland, the adrenal gland sits atop each kidney. The shape can distinguish laterality with the right gland a pyramidal shape whilst the left is more semilunar.
Adrenal cortex
The adrenal cortex is the outermost aspect of the adrenal gland. It is composed of three layers:
- Zona Glomerulosa: mineralocorticoids – aldosterone is the key mineralocorticoid, release primarily controlled by the renin-angiotensin system (described below).
- Zona Fasciculata: glucocorticoids – release controlled by hypothalamic-pituitary-adrenal axis (described below).
- Zona Reticularis: androgens – produces dehydroepiandrosterone (DHEA).
Adrenal medulla
The innermost aspect of the adrenal gland, composed of a single layer. Responsible for the production of three hormones:
- Adrenaline
- Noradrenaline
- Dopamine
Physiology
Under normal conditions aldosterone release is primarily controlled by the renin-angiotensin system.
1. Renin
Renin, a proteolytic enzyme, is released by granular cells of the juxtaglomerular apparatus in response to:
- Renal artery hypotension
- Sympathetic stimulation
- Reduced sodium levels in the distal tubal
In the blood renin cleaves angiotensinogen into angiotensin I.
2. Angiotensin
Angiotensin-converting enzyme (ACE), found primarily in the vascular endothelium of the lungs, cleaves angiotensin I to give angiotensin II.
Angiotensin II has multiple functions:
- Stimulates adrenal cortex to release aldosterone
- Causes vasoconstriction
- Increases sodium reabsorption
- Stimulates the release of anti-diuretic hormone (ADH)
- Increases sympathetic permissiveness
3. Aldosterone
Aldosterone is a mineralocorticoid released from the zona glomerulosa of the adrenal cortex. It is released in response to:
- Angiotensin II (primary stimulus)
- ACTH
- Potassium levels
Its primary action is to increase the number of epithelial sodium channels in the distal tubule. This results in sodium and water reabsorption and potassium excretion.
Pathophysiology
Hypersecretion of aldosterone leads to sodium and water retention and potassium wasting.
Hypertension & hypokalaemia
As described above aldosterone acts on the kidneys, primarily the collecting duct where it increases the number of sodium channels in principal cells. This results in sodium reabsorption followed by water, potassium is excreted as it moves down the electrical gradient.
In the presence of aldosterone excess the human body begins to retain sodium and water. The increase in volume and changes to systemic vascular resistance lead to hypertension. Hypokalaemia may result in 15-30% of cases from the increase in potassium excretion.
Aldosterone escape
The volume expansion prompted by increased water reabsorption results in an ‘escape’ phenomenon. This is a diuresis that occurs in response to the water and sodium retention caused by raised aldosterone. The exact mechanisms are unclear but appear to involve:
- Pressure natriuresis
- Atrial natriuretic peptide release
- Changes to reabsorption in the distal tubule
Aetiology
In the vast majority of cases primary aldosteronism is caused by adrenal adenomas or adrenal hyperplasia.
Bilateral idiopathic hyperplasia
Also called idiopathic adrenal hyperplasia, this the most common cause of primary aldosteronism, implicated in 60-70% of cases. It tends to cause a primary aldosteronism that is less severe than that caused by aldosterone-producing adenomas.
Aldosterone-producing adenoma
Primary aldosteronism was first described by Conn in the 1950’s in patients with adrenal adenomas (hence the alternate name Conn’s syndrome). The autonomous adenoma produces aldosterone independent of normal mechanisms.
Once thought to be the most common cause of primary aldosteronism, it is implicated in around 30-40% of cases.
Unilateral hyperplasia
This is caused by hyperplasia only affecting one gland. Far less common than the bilateral form and may be treated more like an aldosterone-producing adenoma.
Other (rare)
Familial hyperaldosteronism:
- Type 1: Glucocorticoid-remediable aldosteronism
- Type 2: Poorly understood, likely most common familial cause, appears to be due to an inherited adrenal adenoma or idiopathic adrenal hyperplasia.
- Type 3: Mutations in KCNJ5 gene
Adrenal carcinoma is a rare cause.
Clinical features
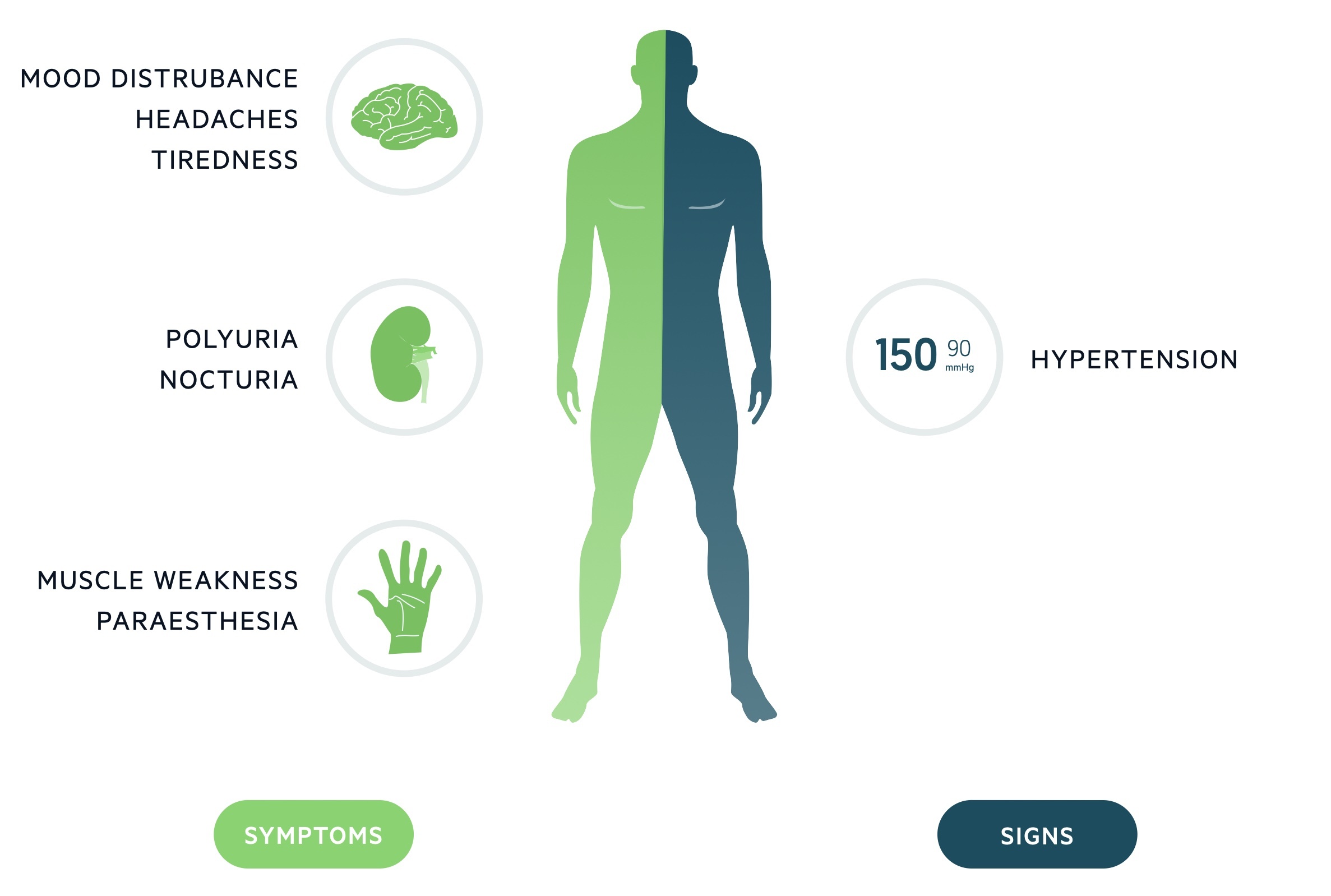
Frequently asymptomatic, clinical features of primary aldosteronism are those of hypokalaemia (30%) and hypertension.
Hypertension
Hypertension is generally asymptomatic but present in the vast majority of patients with primary aldosteronism. Long-standing hypertension may result in end-organ damage:
- Chronic kidney disease
- Cerebrovascular disease
- Heart failure
- Retinopathy
Hypokalaemia
Hypokalaemia affects around 10-30% of patients with primary aldosteronism. It is normally asymptomatic but may present with non-specific signs and symptoms including:
- Muscle weakness
- Paraesthesia
- Mood disturbance
- Polyuria/nocturia
Who to test
Hypertension, hypokalaemia and metabolic alkalosis are the classical findings.
Suggestive findings
Hypertension is essentially universal in primary aldosteronism. All other features are variably present:
- Metabolic alkalosis: caused by increased urinary hydrogen excretion, multifactorial, due to hypokalaemia and direct effects of aldosterone on intercalated cells.
- Hypokalaemia: low serum levels are present in 15-30% of patients. Urinary potassium may demonstrate inappropriate potassium loss in the setting of hypokalaemia.
NOTE: Primary aldosteronism has been described with hypokalaemia but an absence of hypertension – this is normally in young women.
Triggers for testing
Patients should be tested where a secondary cause for hypertension is suspected. This includes:
- Hypertension and hypokalaemia (spontaneous or diuretic-induced)
- Severe hypertension (systolic > 150, diastolic > 100)
- Hypertension resistant to treatment
- Hypertension and:
- Adrenal incidentaloma
- Sleep apnea
- Family history of early onset hypertension
- Family history of early onset CVA
- Primary aldosteronism affecting all 1st degree relatives with hypertension
Diagnosis
Diagnosis of primary aldosteronism requires a multi-tier testing approach.
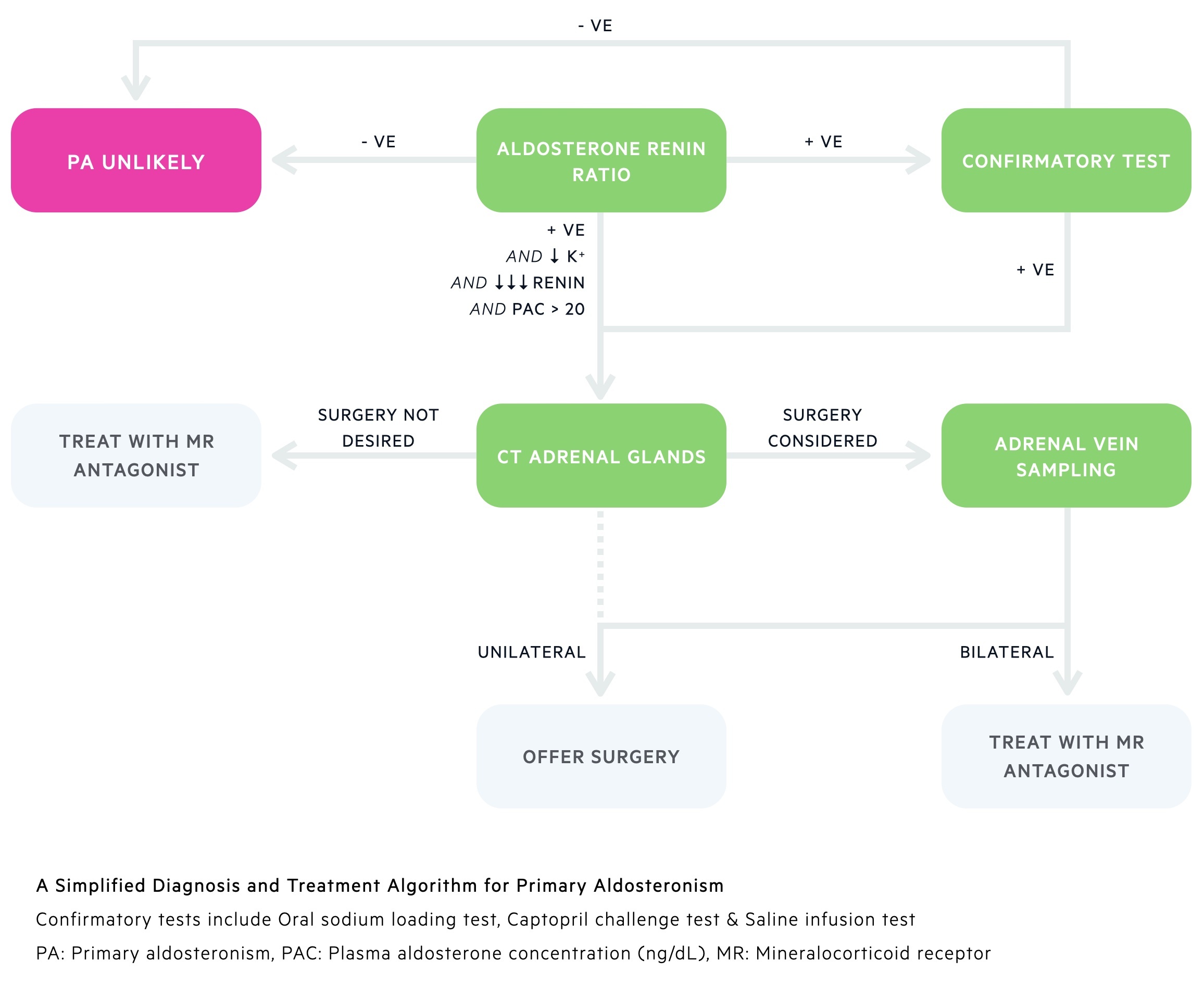
1. Screening
The aldosterone:renin ratio (ARR) is used as a screen in those with suspected primary aldosteronism. It compares serum levels of aldosterone and renin. A raised ratio should prompt further investigation.
2. Confirmatory testing
Those with a raised ARR will normally require confirmatory testing. In patients with positive ARR screening and spontaneous hypokalaemia, undetectable renin and a plasma aldosterone concentration > 20ng/dL confirmatory tests may not be needed.
Where indicated a number of tests are available:
- Oral sodium loading test
- Saline infusion test
- Captopril challenge test
3. Identifying the cause
A CT of the adrenal glands is the next line investigation in people with positive ARR/confirmatory testing. It may provide evidence of an adrenal adenoma or hyperplasia. CT can be misleading and it is at times difficult to distinguish adenomas from non-functioning incidentalomas or even hyperplasia based disease.
The gold-standard to confirm laterality is adrenal vein sampling – it has an excellent ability to differentiate between bilateral and unilateral disease. It is however an invasive and technically difficult procedure with risk of bleeding, thromboembolism and contrast reactions. It should not be performed in patients unwilling or not fit for surgery.
Investigations and management are guided by expert MDTs. They may consider avoiding AVS and proceeding to unilateral adrenalectomy in select patients – typically young patients with frank primary aldosteronism and findings consistent with an adenoma on CT.
Management
Management of primary aldosteronism involves both surgical and medical modalities.
Unilateral disease
This includes patients with aldosterone-producing adenomas and unilateral hyperplasia. In those whom are fit and willing, unilateral adrenalectomy offers cure. This may be either laparoscopic or open depending on patient factors and local expertise.
Medical management with mineralocorticoid receptor antagonists (e.g. spironolactone) can be used as a bridge to surgery or as definitive management in those not for surgical intervention.
Bilateral disease
Medical management is typically used:
- Mineralocorticoid receptor antagonists: Spironolactone, Eplerenone (newer, unlike spironolactone does not cause gynaecomastia).
- ENaC inhibitor: Amiloride, a potassium-sparing diuretic, may be used if aldosterone antagonists are not tolerated.
Other antihypertensives may be added.

