Overview
Diabetes mellitus refers to a group of metabolic disorders that result from an inability to produce and/or reduced sensitivity to insulin.
Diabetes mellitus
Diabetes mellitus is a chronic, multi-system disease, with profound biochemical and structural sequelae. It can be classified into four main groups:
- Type 1 diabetes mellitus: characterised by an inability to produce/secrete insulin due to autoimmune destruction of the beta-cells (production site of insulin) in the pancreatic islets of Langerhans.
- Type 2 diabetes mellitus: characterised by a combination of peripheral insulin resistance and inadequate secretion of insulin. It is strongly associated with obesity and the metabolic syndrome.
- Gestational diabetes mellitus: new onset of diabetes in pregnancy. It is associated with both maternal and foetal complications and as such patients are managed as part of a multi-disciplinary team in both antenatal and diabetic clinics. Patients with GDM have a higher risk of developing both GDM in future pregnancies and overt diabetes mellitus.
- Other: These can be divided into genetic and acquired disease. Genetic causes refer to monogenic diabetes (i.e caused by mutation to a single gene). They are rare and collectively termed ‘mature-onset diabetes of the young’ (MODY). Acquired causes may be secondary to medications or pathological conditions. Common causes include corticosteroids, pancreatitis and pancreatic tumours.
T2DM
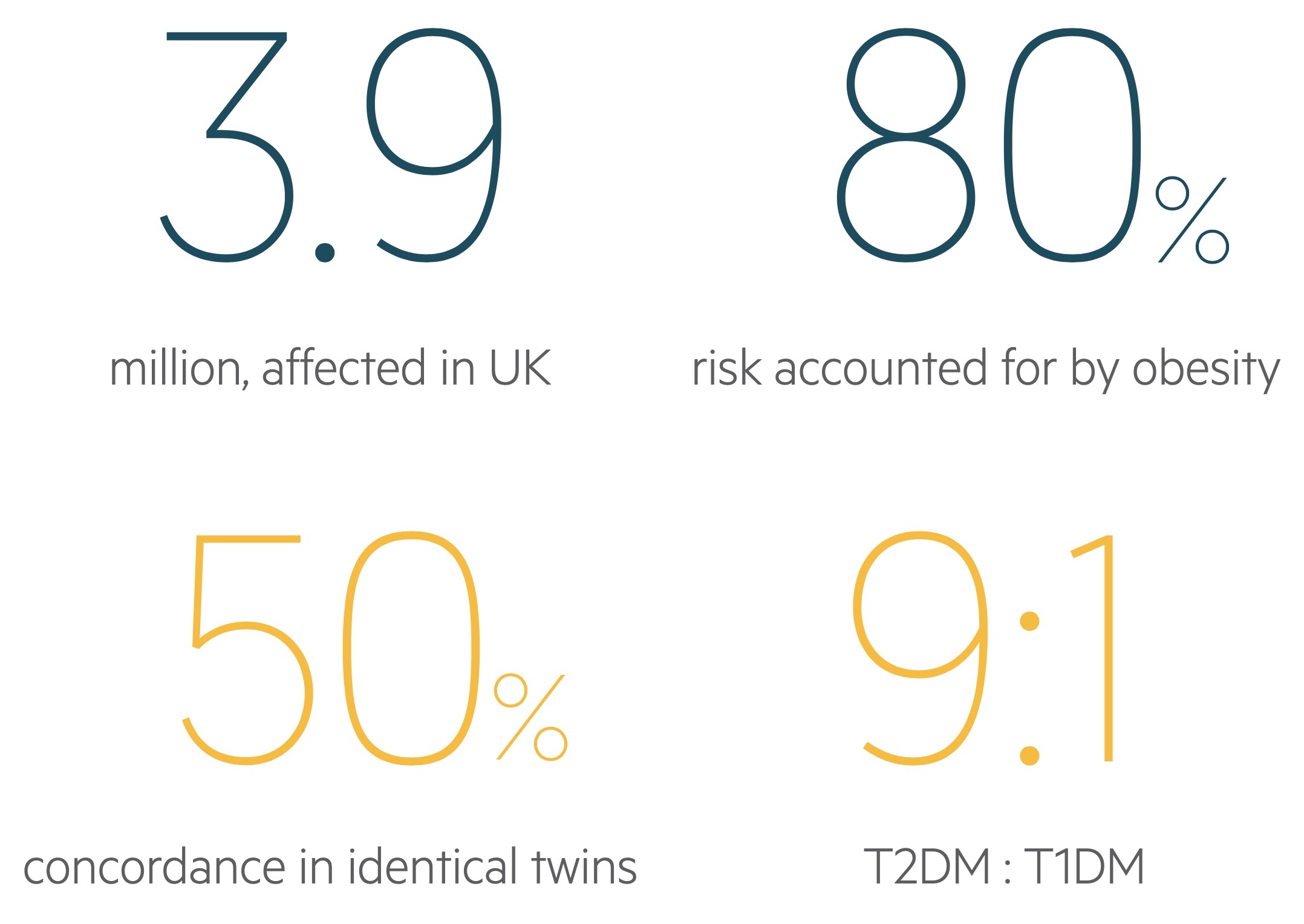
Type 2 diabetes is a condition caused by a combination of insulin resistance and deficiency. It is the most common form of diabetes and is responsible for a huge amount of morbidity and mortality in the UK.
Aetiology
T2DM is a complex, polygenic disease that is strongly associated with obesity.
Development of the condition is dependent on the interaction between the genetic make-up of the patient and their environment. Approximately 80% of the risk for developing T2DM is due to obesity.
T2DM is a worldwide public health concern with over 500 million people expected to suffer from the condition by 2030, over half being unaware of their condition.
Genetic risk
T2DM has been shown to have a genetic basis. The risk of developing the condition is as high as 75% if both parents have suffered from the condition. Moreover, in monozygotic twins, there is a 50-90% concordance for developing the condition.
Importantly, T2DM is considered a polygenic disease with numerous genetic variants contributing to overall disease risk. Certain ethnicities (e.g. Asian/African) have a 2-4x increased risk of developing the condition.
Environmental risk
Environmental risk factors have a significant impact on the development of T2DM. Obesity and inactivity account for the majority of this risk (80-85%). Obesity is associated with insulin resistance and the metabolic syndrome.
Other major environmental risk factors include:
- Poor dietary habit (low fibre, high glycemic index diet)
- Low birth weight
- Medications
- Polycystic ovarian syndrome
- History of GDM
Pathophysiology
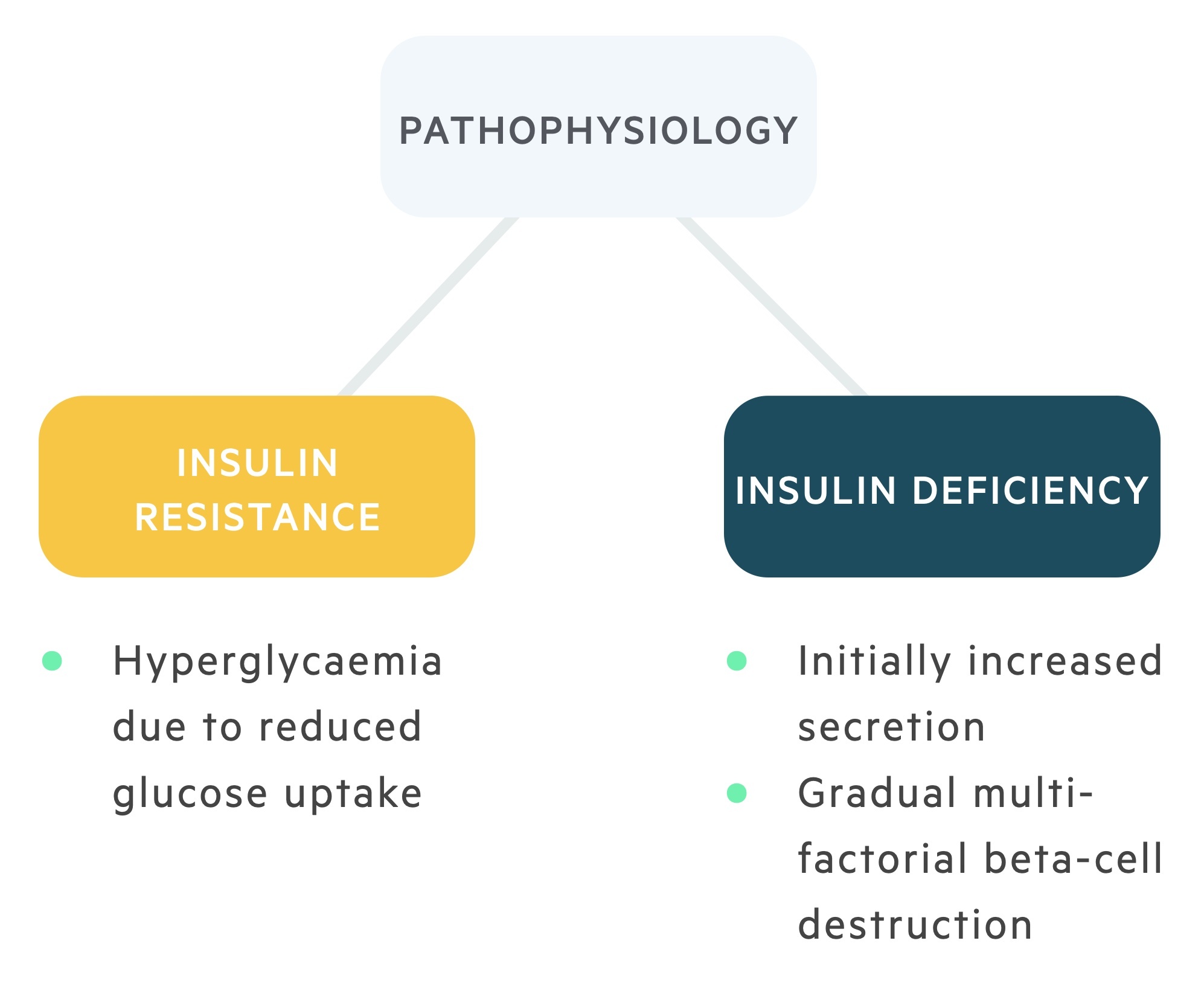
The two principle mechanisms involved in the development of T2DM are peripheral insulin resistance and inadequate insulin secretion.
It has long stood that peripheral insulin resistance was at the centre of T2DM. The importance of reduced insulin secretion and beta-cell dysfunction in the pathophysiology is now acknowledged.
Insulin resistance
During normal control of glucose homeostasis, the release of insulin following ingestion of a meal leads to the uptake of glucose into cells (peripheral muscle).
Insulin is able to bind to insulin receptors on the cell surface. Binding leads to a number of downstream responses including effects on lipid metabolism, protein metabolism and cellular growth. In general, these are anabolic effects that:
- Inhibit proteolysis and lipolysis
- Inhibit hepatic gluconeogenesis
- Promotes hepatic glycogen synthesis
Activation of the insulin receptor also initiates translocation of the glucose receptor GLUT-4 (found in the cytosol of adipose and striated muscle) to the cell surface. This allows movement of glucose intracellularly.
Resistance to insulin causes disruption of these metabolic processes. Hyperglycaemia develops due to failed glucose uptake and there are catabolic effects on lipids (increased lipolysis), protein (increased proteolysis) and hepatic tissue (increased hepatic gluconeogenesis and reduced hepatic glycogen synthesis). The exact mechanism involved in insulin resistance is poorly characterised.
An inability of peripheral tissue to respond to insulin leads to hyperglycaemia, which in turn causes hyperinsulinaemia. In the early stages of insulin resistance, abnormal regulation of glucose occurs following a meal in the post-prandial state. This is termed impaired glucose tolerance (IGT). IGT is a major risk for the future development of frank diabetes and cardiovascular disease.
High levels of glucose during the fasting state (between meals) can also occur with insulin resistance, which is termed impaired fasting glucose (IFG). IFG itself is another risk factor for the development of frank diabetes. IFG and IGT are often collectively referred to as ‘pre-diabetes’ as they are significant markers for the future development of the condition if no intervention occurs to reduce risk (e.g. lifestyle and diet modifications).
Insulin secretion
A reduction in insulin secretion is now central to the pathogenesis of T2DM with the development of beta cell dysfunction and gradual loss of beta cell mass.
As hyperglycaemia develops, the beta cells within the Islets of Langerhans must secrete more insulin to deal with the increase in glucose leading to hyperinsulinaemia. These high levels of insulin are still inadequate to restore glucose homeostasis. It is thought that high glucose levels (and lipids) are toxic to beta cells leading to a depletion in their cellular mass. This is termed glucotoxicity. During autopsy, patients with T2DM have shown to have deposition of amyloid within islet cells supporting the idea of beta cell depletion.
As a significant proportion of the beta cell mass is depleted, insulin levels begin to decline and there is secretary failure. This may progress toward absolute insulin deficiency, loss of glucose control, and requirement of insulin. The time frame for the development of beta cell dysfunction to absolute insulin deficiency is highly variable among patients.
Clinical features
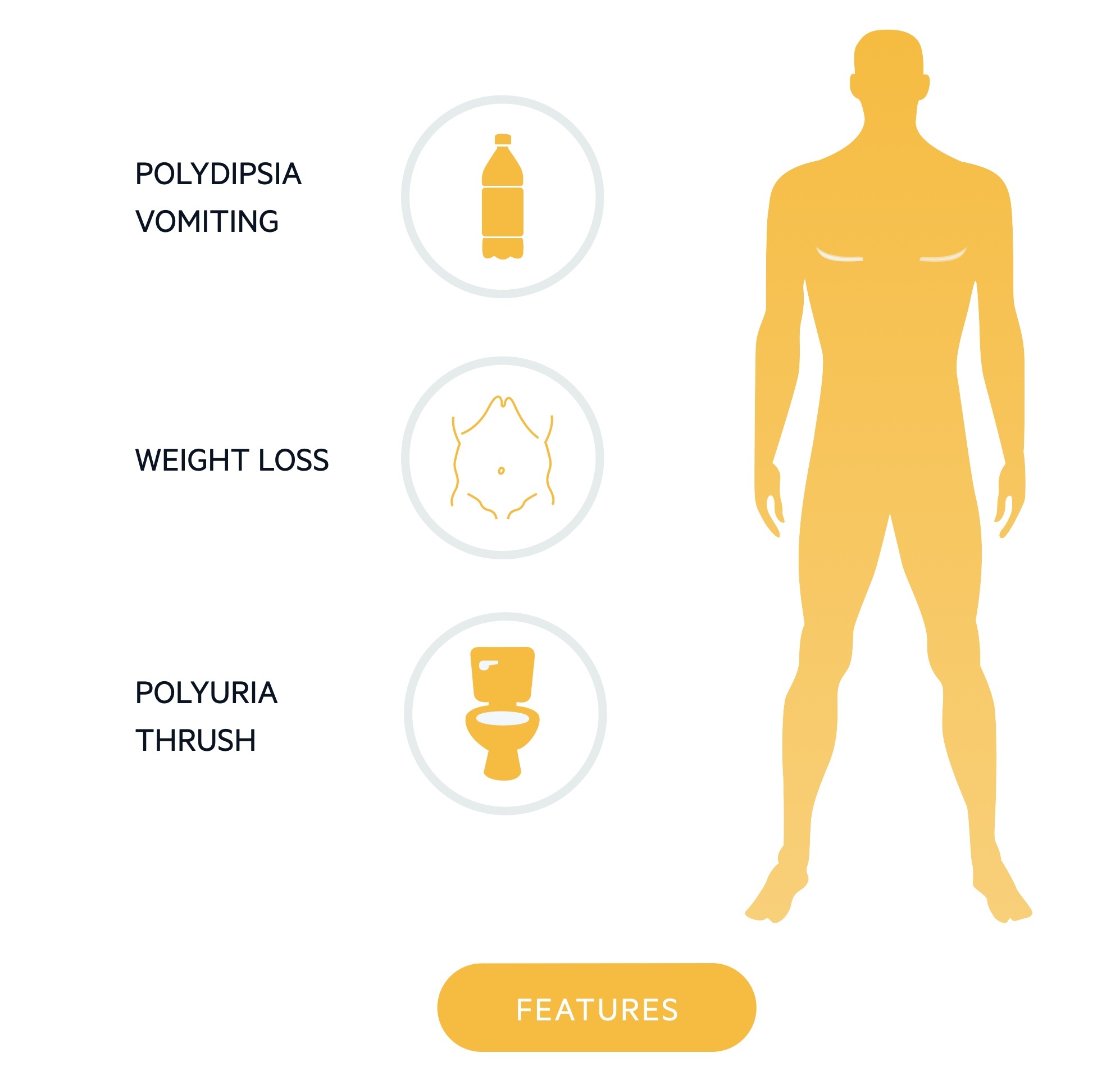
Patients may complain of lethargy, polyuria, polydipsia, weight loss, and recurrent infections (e.g. thrush, balanitis) or be picked up on routine testing.
NOTE: Diabetes mellitus is a chronic, multi-system disease that has profound biochemical and structural sequelae. In patients with established chronic disease, it is important to consider clinical features associated with the variety of microvascular and macrovascular complications that can develop. These are discussed further below.
Investigations & diagnosis
The major diagnostic tool in T2DM is the measurement of glycated haemoglobin (HbA1c).
Other tests that can be used in the diagnosis and assessment of T2DM include random plasma glucose levels, fasting plasma glucose levels and an oral glucose tolerance test (OGTT).
HbA1c
HbA1c (glycated haemoglobin) is measured in the blood and reflects the average blood glucose concentration over a three month period (the average survival time of an erythrocyte). Glycated haemoglobin occurs due to non-enzymatic irreversible modification of the beta globin chain in haemoglobin. As blood glucose levels increase the amount of glycation of haemoglobin also increases.
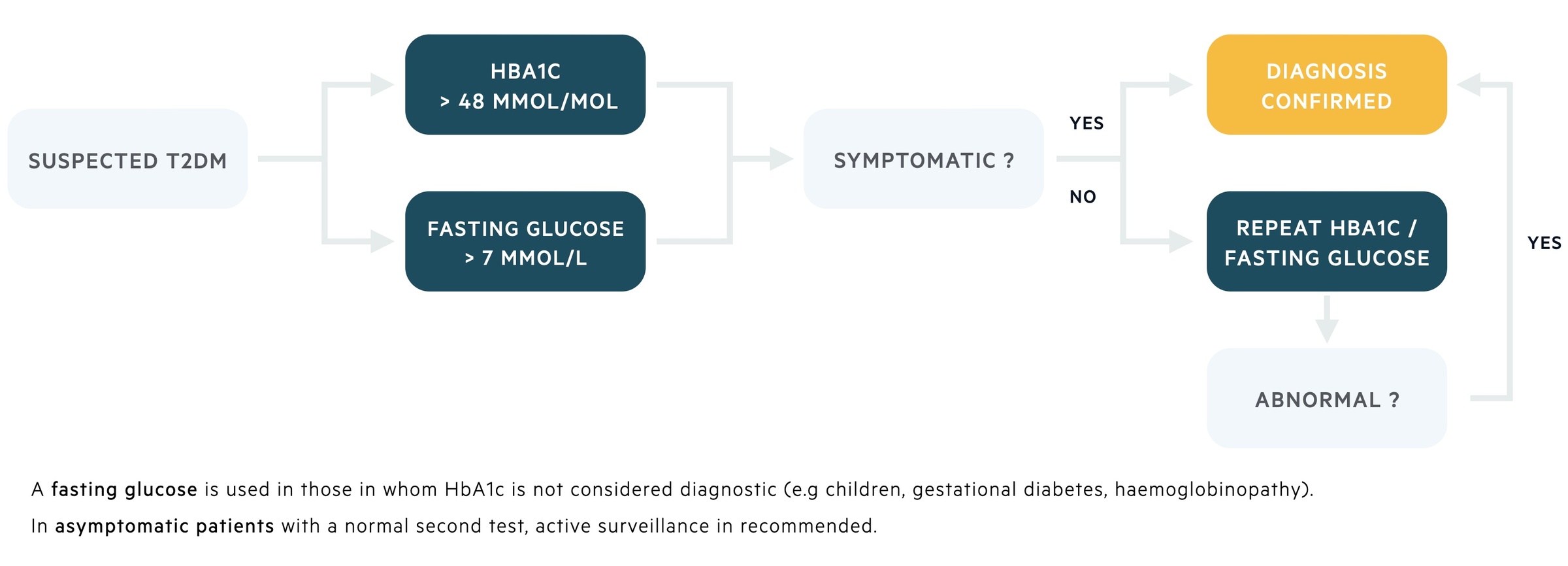
In general, the diagnosis of diabetes is made if the level of HbA1c is ≥ 48 mmol/mol (6.5%), which correlates with an average blood glucose level of 7.8 mmol/L. Patients with a level between 42-47 mmol/mol (6-6.4%) are at risk of developing diabetes and said to be ‘pre-diabetic’. Importantly, the use of HbA1c as a diagnostic tool is dependent on whether the patient is symptomatic.
If the patient is symptomatic (e.g. thirst, polyuria), then a single HbA1c reading of 48 mmol/mol or greater is enough to confirm the diagnosis. If the patient is asymptomatic, the diagnosis should be based on two abnormal readings (either two HbA1c readings, or one HbA1c reading and an additional test such as fasting glucose levels).
There are some situations in which HbA1c should be used with caution due to other co-morbidities. In general, conditions interrupting erythropoiesis (e.g. EPO use, iron-deficiency), haemoglobin structure (e.g. haemoglobinopathies), glycation (e.g. CKD, alcoholism), or red cell survival (e.g. haemolysis, splenectomy) will effect the level of HbA1c. In these situations, the HbA1c should be used with caution or an alternative test used (e.g. fasting glucose).
Fasting plasma glucose levels
Normal fasting glucose levels should be approximately 4-6 mmol/L.
The diagnosis of diabetes can be made if the fasting glucose levels are ≥ 7 mmol/L on two separate readings in a patient who is asymptomatic or a single reading if the patient is symptomatic. As discussed above, ‘two readings’ may be two fasting glucose measurements or one fasting glucose measurement and one HbA1c.
Patients with a fasting glucose level between 6-6.9 mmol/L are said to have impaired fasting glucose (IFG), which is a marker of pre-diabetes and increases the risk of developing overt diabetes.
Random plasma glucose levels
A random blood glucose level ≥ 11.1 mmol/L is indicative of diabetes. This measurement is not currently used in the diagnosis of the condition.
Patients with a high random plasma glucose should be assessed for potential complications (e.g. HHS, DKA) and concurrent illness. Concurrent illnesses can increase plasma glucose levels as part of the normal physiological stress response. Patients with high levels should undergo further testing (e.g. HbA1c or fasting glucose levels).
Oral glucose tolerance test
The oral glucose tolerance test (OGTT) measures the ability of the body to deal with a glucose load over a two hour period. Its use has largely been replaced by HbA1c in clinical practice.
The OGTT firstly involves the patient fasting (usually overnight). A glucose measurement can then be taken at time 0 hrs. The patient is then given a 75 g glucose load. Traditionally, a blood sample for glucose is then taken at 2 hours. Levels greater than 11 mmol/L at 2 hours are suggestive of diabetes. Levels ≥ 7.8 mmol/L but < 11.1 mmol/L are suggestive of impaired glucose tolerance (IGT).
The OGTT is usually reserved for assessment of gestational diabetes. It should be noted that NICE produced updated guidelines on the diagnosis of gestational diabetes where the values for diagnosis are set much lower than in non-pregnant patients.
Management
The management of T2DM requires education, lifestyle changes, medications and the monitoring of complications.
The management of type 2 diabetes mellitus has become more complicated in recent years with the development of newer drugs and the increasing use of insulin therapy.
Where appropriate, management of diabetes should be individualised to the patient to try and achieve the best possible glycaemic control to prevent long-term sequelae without causing significant side-effects or reduced quality of life.
Here, we will discuss some of the essential aspects to the management of T2DM:
- Lifestyle advice
- Antidiabetic drugs
- Insulin use in T2DM
- Treatment targets
- Monitoring for complications
Lifestyle advice
Lifestyle changes are crucial to all aspects of management in patients with T2DM. They predominantly refer to dietary changes, exercise and physical activity, alcohol consumption and smoking cessation.
Lifestyle modifications may be the earliest changes instigated in patients with or at risk of developing diabetes (e.g. HbA1c 42-47 mmol/mol). It is essential that these modifications continue once antidiabetic medications are introduced. Lifestyle advice can help patients achieve sustained weight loss, better physical fitness, improved diet and ultimately improved glycaemic control.
Patients should be encouraged to maintain a healthy balanced diet with plenty of fibre, low-index carbohydrate and controlling the intake of high-fat foods. In those with excess body weight, dietary plans can help with weight loss, targeted plans may be appropriate.
Exercise can lower blood glucose levels as it increases glucose use by muscles. Patients should be informed about the UK physical fitness guidelines and encouraged to exercise daily with at least 150 minutes of moderate intensity activity over a weekly period.
Patients should be encouraged to reduce alcohol consumption and stop smoking. Alcohol increases weight and may exacerbate or prolong hypoglycaemia induced by antidiabetic medications. Smoking cessation advice should be made available and offered to all patients with T2DM.
Antidiabetic drugs
There are a significant number of antidiabetic medications that are now available for the treatment of T2DM. The choice of treatment and intensity of therapy should take into account the individual patients needs, comorbidities, the ability to achieve a HbA1c target, tolerability of treatment and potential side-effects.
Metformin is one of the most commonly used medications in T2DM. Metformin is a biguanide, which lowers blood glucose levels through inhibition of hepatic gluconeogenesis whilst increasing peripheral insulin sensitivity and enhancing peripheral uptake of glucose. All patients with T2DM should be started on metformin unless contraindicated (e.g. CKD, those at risk of lactic acidosis, poorly tolerated).
Below is a summary of the current treatment algorithm for patients with T2DM. Please note the precise mechanism of all antidiabetic medications is beyond the scope of these notes.
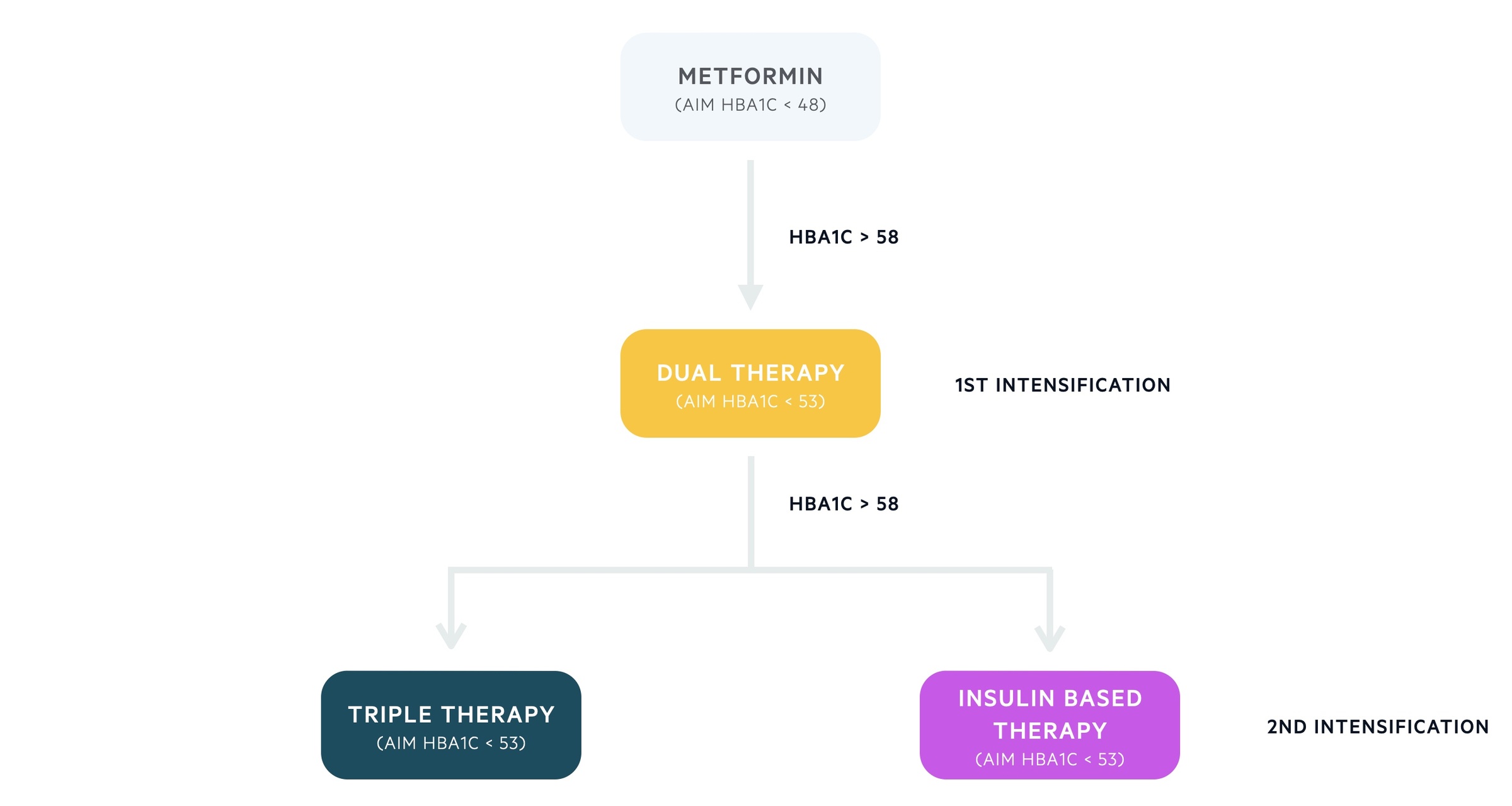
Step 1
- Standard release metformin
- Aim for HbA1c < 48 mmol/mol, increasing dose as needed
- Monitor renal function, consider modified-release preparations if develop adverse GI effects
Step 2 (first intensification)
- Consider dual antidiabetic therapy if HbA1c rises > 58 mmol/L
- Metformin in combination with a second antidiabetic agent:
- Sulfonylurea (SU)
- Dipeptidyl peptidase-4 inhibitor (DPP-4i)
- Pioglitazone
- Sodium–glucose cotransporter 2 inhibitor (SGLT-2i)
- Aim for HbA1c < 53 mmol/mol
Step 3 (second intensification)
- Consider triple antidiabetic therapy or an insulin-based regimen if HbA1c > 58 mmol/mol
- Metformin, DPP-4i and SU
- Metformin, pioglitazone and SU
- Metformin, pioglitazone/SU, and SGLT-2i
- Insulin-based regimes are discussed further below
Step 4
- In patients on triple antidiabetic therapy and ongoing poor glucose control, consider further intensification
- Consider combination treatment with metformin, SU and glucagon-like peptide-1 (GLP-1) mimetic
- It should be noted there are strict criteria for the use of a GLP-1 analogue and patients must achieve certain glycemic and weight targets to warrant continuing the medication
- If already on an insulin-based regime, seek specialist advice
If metformin is contraindicated an alternative treatment ladder is used.
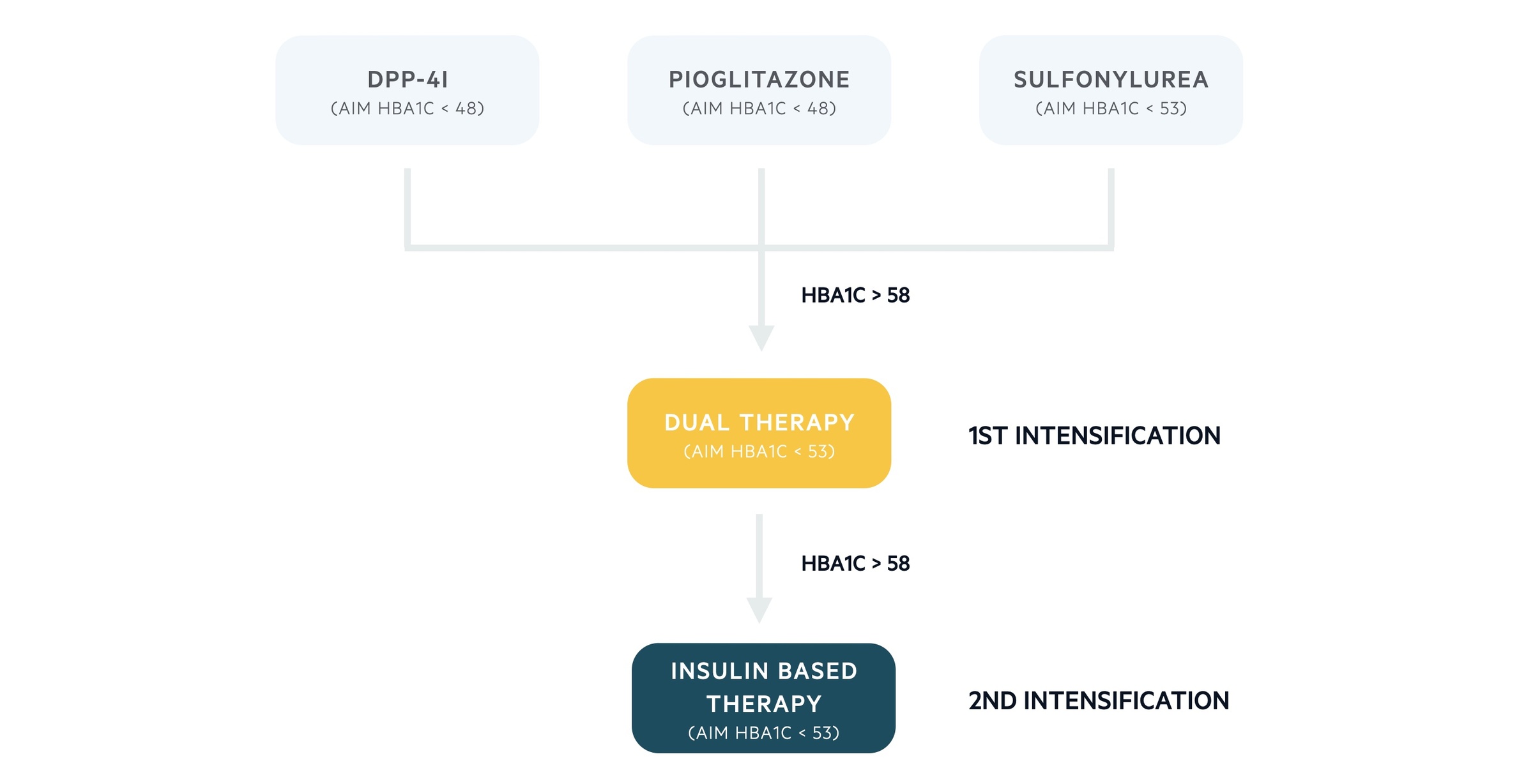
Alternative step 1
- DPP-4i or pioglitazone or SU
- Aim for HbA1c < 48 mmol/mol or < 53 mmol/mol if treatment with a SU
Alternative step 2 (first intensification)
- If HbA1c rises > 58 mmol/mol consider a combination of:
- SU and pioglitazone
- SU and DPP-4i or
- Pioglitazone and DPP-4i
Alternative step 3 (second intensification)
- Consider an insulin-based regimen if HbA1c > 58 mmol/mol
Insulin-based regimens
The use of insulin should be considered early in the management of patients with T2DM. It is usually considered in patents with poor glycemic control despite dual antidiabetic with metformin and another agent.
Insulin therapy may not be appropriate for those at significant risk of hypoglycaemia, a personal preference against insulin or concerns relating to licensing for driving group 2 vehicles. Obese patients may find insulin may exacerbate weight issues, its use should be avoided where possible.
Insulin therapy should be initiated by healthcare professionals experienced with the use of insulin. There are a number of insulin regimes that may be used in patients with T2DM:
- Once or twice daily intermediate-acting insulin (NPH)
- Intermediate-acting insulin along-side a short-acting insulin as separate injections or a pre-mixed formula
- Once daily long-acting insulin therapy (glargine, determir)
- Basal-bolus regimes
In general, patients are offered an intermediate-acting insulin therapy such as NPH in the initial stages. If glycemic control is particularly bad (e.g. HbA1c > 75 mmol/L) patients may be started on mixed insulin (intermediate and short-acting). Long-acting insulin is usually reserved for patients where hypoglycaemia is a problem with NPH insulin or the patients rely on carers for help with their injections. Finally, patients on a pre-mixed insulin regime that still have inadequate blood glucose control should be assessed for conversion to a basal bolus regime.
There are many other facets to starting patients on insulin therapy such as equipment use, blood glucose monitoring, sick-day rules and rules for driving. These aspects of care are beyond the scope of these notes.
Treatment targets
Treatment targets for patients with T2DM are largely based on serial measurements of HbA1c.
Patients with poor glycaemic control require measurement of the HbA1c every 3 months. Patients with stable disease can have their HbA1c measured every 6 months.
The main treatment targets are shown below:
- Management with lifestyle modifications only: aim HbA1c < 48 mmol/mol
- Management with lifestyle and a single antidiabetic agent: aim HbA1c < 48 mmol/mol
- Management with a drug associated with hypoglycaemia (e.g. SU): aim HbA1c < 53 mmol/mol
- Management with higher intensification regimes: aim HbA1c < 53 mmol/mol
If HbA1c levels are not adequately controlled by a single drug and rise to ≥ 58 mmol/mol, then intensification of medical therapy should be considered. Additionally, reinforcement about dietary advice, lifestyle changes, adherence to medical treatment should be completed to support patients in achieving a HbA1c < 53 mmol/mol.
It is worth noting that where possible, treatment targets should be individualised to the patient to help them with the long-term management of their condition. HbA1c targets may be relaxed due to the risk of hypoglycaemia, reduced life-expectancy or minimal long-term benefit.
Monitoring for complications
T2DM is a systemic condition that is associated with a number of complications that can have a significant impact on morbidity and mortality. As such, patients should be regularly reviewed and complications assessed on at least an annual basis.
During each clinic review, patients should have their BMI checked, smoking status clarified and assessed for any psychosocial issues (e.g. anxiety/depression). Every 3-6 months, patients should have their HbA1c checked and management changed accordingly (discussed above). Finally, on annual basis patients should be screened for major complications of diabetes:
- Retinopathy: annual retinal screening and eye check
- Nephropathy: renal function (eGFR) and albumin:creatinine ratio (ACR)
- Neuropathy and diabetic foot: assessment for peripheral neuropathy, autonomic neuropathy, full examination including footwear, monofilament assessment of neuropathy, vascular assessment +/- dopplers.
- Cardiovascular risk factors: primary/secondary prevention strategy with optimisation of blood pressure and lipids.
Acute complications
The two major acute diabetic complications seen in patients with T2DM are hypoglycaemia and hyperosmolar hyperglycaemic state (HHS).
For our notes on these topics click below:
- Hypoglycaemia
- Hyperosmolar hyperglycaemic state
It is worth noting that some patients with T2DM are also at risk of developing diabetic ketoacidosis (DKA) due to beta cell dysfunction and a lack of insulin secretion. Patients presenting unwell with type 2 diabetes mellitus should be assessed for DKA (e.g. urinary/plasma ketone levels, pH and bicarbonate) and treated accordingly. Both HHS and DKA are associated with significant mortality if not treated promptly and the reasons for developing these conditions should be explored with the patient during admission (i.e. non-adherence to treatment, intercurrent illness).
Microvascular complications
Uncontrolled diabetes results in small vessel disease affecting the kidneys, nerves and retinas (amongst other systems).
Retinopathy
Diabetic retinopathy is one of the major eye-related disorders that can occur in patients with diabetes. More than 95% of patients will have detectable changes after 20 years. Other disorders include cataracts and ocular palsies. When diagnosed with T2DM, GPs should immediately refer for eye screening.
Persistent damage to the retina leads to areas of ischaemia and release of angiogenetic factors such as vascular endothelial growth factor (VEGF). This promotes new formation of vessels that are weak and friable. This leads to complications including haemorrhage, fibrosis and retinal detachment.
There are a number of classification systems, here we will use the NSC-UK classification.
- Non-proliferative:
- Background (R1): dot and blot haemorrhages, hard exudates, cotton wool spots
- Pre-proliferative (R2): intraretinal microvascular abnormalities (IRMA), venous beading
- Proliferative:
- Proliferative (R3): new vessels at the disc and elsewhere (NVD, NVE), fibrosis, traction retinal detachment
- Maculopathy: exudates, oedema, NVE
Good glycemic control and regular screening to assess early changes are key to preventing and managing disease. Photocoagulation is used to manage proliferative disease. The aim of photocoagulation is to burn holes within the ischaemic retina to prevent the release angiogenesis factors (e.g. VEGF), which stimulate new vessel formation.
Nephropathy
Diabetic nephropathy is one of the most common causes of chronic and end-stage kidney disease.
The earliest sign of diabetic nephropathy is the presence of microalbuminuria, which can be assessed with an albumin:creatinine ratio (ACR). An ACR 3 – 30 mg/mmol is suggestive of microalbuminuria, now categorised as moderately increased albuminuria.
A1: < 3 mg/mmol, normal or mild increase
A2: 3 – 30 mg/mmol, moderately increased
A3: > 30 mg/mmol, severely increased
Microalbuminuria is a marker of systemic microvascular damage and patients should be treated with an ACE inhibitor on angiotensin receptor blocker, even in the presence of normotension. Chronic kidney disease (CKD) in diabetes is evidenced by a persistently low eGFR < 60 mmol/L and/or an ACR persistently > 3 mg/mmol/L.
Additional considerations are now recommended for the prescription of sodium-glucose transport protein 2 (SGLT2) inhibitors to patients with T2DM and CKD:
- Consider an SGLT2 if ACR 3-30 mg/mmol
- Offer an SGLT2 if ACR > 30 mg/mmol
Neuropathy
Diabetic neuropathy refers to a collection of conditions that result from glucose-related damage to neurones of the somatic and autonomic nervous systems.
The main types of diabetic neuropathy are listed below.
- Symmetrical polyneuropathy: typically a peripheral neuropathy that occurs in the leg secondary to loss of vibration, pain and temperature sensation.
- Mononeuropathy: damage to a single cranial or peripheral nerve (e.g. third nerve palsy).
- Diabetic amyotrophy: a spectrum of disease affecting the lumbosacral plexus leading to symmetrical pain, weakness and wasting of the proximal muscles of the leg.
- Autonomic neuropathy: a spectrum of conditions related to damage of the autonomic nervous system, which can affect multiple systems.
Autonomic complications of diabetes can have quite profound effects on patients and may require multi-disciplinary input into their care. Some of the major autonomic neuropathies include postural hypotension, gastroparesis (delayed gastric emptying leading to vomiting), diarrhoea, bladder dysfunction and erectile dysfunction.
Diabetic foot
Diabetic foot problems refer to a wide range of pathologies that are thought to occur secondary to the combination of peripheral neuropathy and poor vascular supply.
Due to loss of sensation and poor blood supply, patients are at risk of a number of complications including diabetic ulcers, secondary infection (e.g. cellulitis, osteomyelitis), skin necrosis and eventually amputation.
Another well-known problem is Charcot’s joint. This is a complex neuropathic arthropathy that results from loss of sensation and subsequent repeated micro-trauma to the foot (traditionally the mid-foot). Microtrauma in the presence of poor peripheral blood flow leads to remodelling, swelling and distortion of the whole joint.
It is estimated that 10-15% of diabetic patients will develop a foot ulcer during their illness and approximately 50% of diabetic hospital admissions are related to diabetic ulcers and foot problems. The management of diabetic foot disease is essential to help recognise and treat complications early. There are now recognised diabetic foot services that are available to help manage patients with these conditions.
Macrovascular complications
Diabetes is a significant risk factor for atherosclerosis and related macrovascular complications.
Optimisation of cardiovascular risk factors on an annual basis is essential to prevent premature cardiovascular disease. This involves:
- Smoking cessation
- Nutritional support & exercise
- Consideration of anti-lipid therapy (e.g. atorvastatin)
- Blood pressure control (e.g. ACE inhibitor)
For further information on the management of macrovascular complications including indications for drug therapy, management targets and referral pathways, please see the NICE guidance on the management of type 2 diabetes mellitus.


