Definition & classification
Hyperglycaemic hyperosmolar state (HHS) is an acute diabetic emergency that occurs in patients with type 2 diabetes mellitus.
HHS occurs insidiously over several days with dehydration and metabolic disturbances that are more extreme than diabetic ketoacidosis (DKA).
It is characterised by:
- Hypovolaemia
- Hyperglycaemia (> 30 mmol/L)
- Mild or absent ketonaemia (blood ketones < 3 mmol/L)
- High osmolality (> 320 mOsm/kg)
HHS is clinically distinct to DKA and requires a different approach to treatment. Patients are usually elderly with multiple co-morbidities, and as a result may be very unwell.
The condition was previously termed Hyperglycaemic Hyperosmolar Non-ketotic Coma (HONK). The terminology was altered as it was felt that most patients did not present in a comatose state, but extremely unwell.
Epidemiology
HHS is a life-threatening condition, which usually occurs in the elderly but is increasingly recognised in younger patients.
The incidence is difficult to calculate, but it estimated that HHS accounts for only 1% of diabetic hospital admissions. The average age of presentation is 60 years old and it is associated with a 15-20% mortality.
HHS is often the first the presentation of type 2 diabetes mellitus in up to 20-30% of cases.
Pathophysiology
In HHS, the relative lack of insulin is coupled with a rise in counter-regulatory hormones (e.g. cortisol, growth hormone, glucagon) that leads to a profound rise in glucose.
These patients retain a certain level of insulin, which prevents the development of ketosis that epitomises DKA. However, the level of insulin is inadequate to prevent profound hyperglycaemia.
The excessive glucose leads to massive osmotic diuresis within the kidneys with the loss of essential electrolytes such as sodium and potassium. This is because the proximal tubules within the kidneys only have a certain capacity for reabsorption of glucose. Once this is reached, the remaining glucose is passed through the renal nephrons causing diuresis.
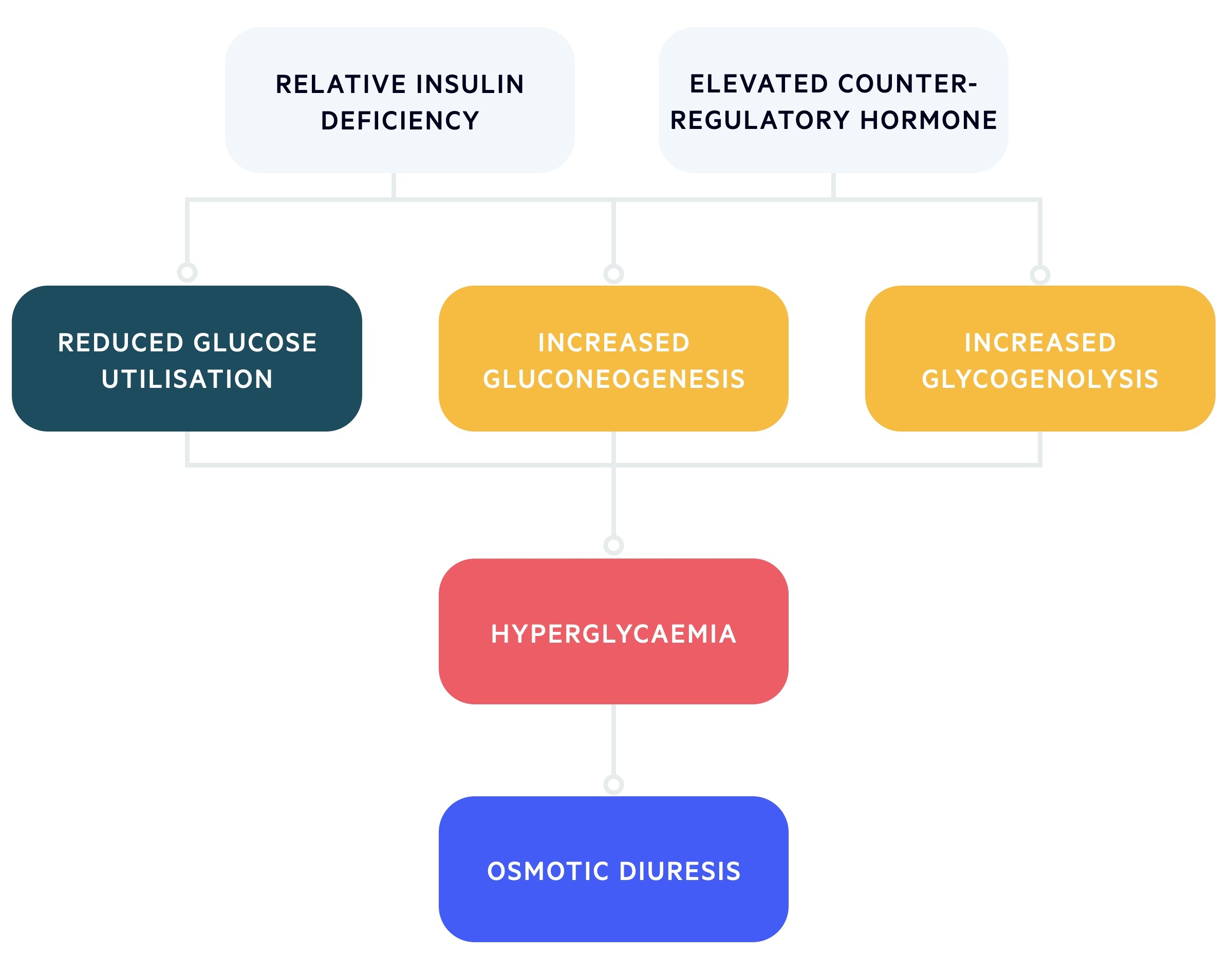
As water is lost, there is profound dehydration and reduced circulating volume, resulting in hyperosmolarity and marked hyperglycaemia. Patients with HHS may have up to a 9 litre deficit of water.
The increase in osmolality increases compensatory mechanisms such as release of anti-diuretic hormone (ADH) and stimulation of thirst. However, if this cannot compensate for the renal water loss (e.g. elderly patients with co-morbidities) then hypovolaemia develops with progression to acute kidney injury, electrolyte disturbances, hypotension and coma.
The hyperosmolar state of the condition leads to hyperviscosity that increases the risk of arterial and venous thrombosis (e.g. stroke, DVT).
Precipitants
A number of underlying conditions are known to precipitate development of HHS, although most cases represent a new diagnosis of type 2 diabetes mellitus.
Common precipitants of HHS include:
- Infection
- High-dose steroids
- Myocardial infarction
- Vomiting
- Stroke
- Poor treatment concordance
Clinical features
Onset of HHS is usually insidious with development of increased renal water loss and dehydration over days to weeks.
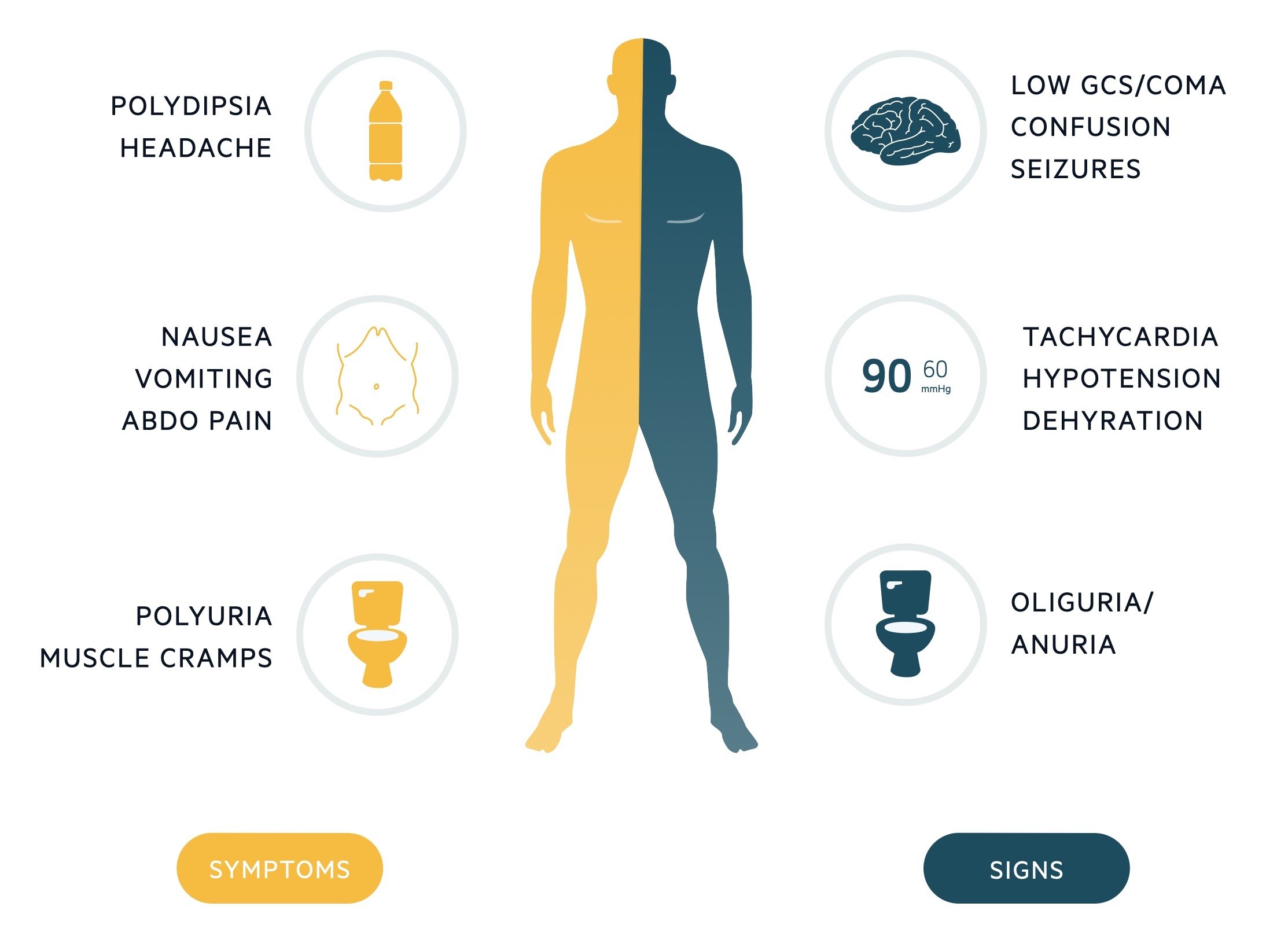
Early features may include polyuria, polydipsia and nausea, whereas late features typically include altered mental status, seizures and possibly coma.
Symptoms
- Polydipsia
- Polyuria
- Nausea
- Vomiting
- Muscle cramps
- Weakness
- Altered mental status
- Seizures
- Coma
Signs
- Dehydration (dry mucous membranes, sunken eyes, reduced capillary refill, decreased skin turgor)
- Hypotension
- Decreased urine output
- Decreased conscious level
- Coma
- Focal neurology signs
- Features of the precipitating cause
Diagnosis
The diagnosis of HHS is based on identification of characteristic features including marked hyperglycaemia, raised serum osmolality, and mild/absent ketonaemia.
Immediate investigations to establish diagnosis of HHS:
- Laboratory glucose: > 30 mmol/L
- Serum osmolality: > 320 mOsm/kg
- Ketones:
- Urine: 1+, trace, negative OR
- Blood: < 3 mmol/L
These laboratory values should be taken in context with the clinical state of the patient (i.e. presence of hypovolaemia/dehydration).
In the absence of a laboratory value for plasma osmolality, the following formula can be used as a surrogate marker for osmolality: Osmolality = 2Na + Urea + Glucose.
Investigations
The key investigations for management of HHS include a laboratory glucose, urea & electrolytes blood test, a blood gas (venous/arterial) and a blood or urinary ketone level.
Additional tests are required for a proper assessment of the patient looking for any precipitating cause of the condition and for complications.
Bedside tests
- ECG
- Urinalysis +/- MSU
- Urinary pregnancy test
Blood tests
- FBC
- U&Es
- CRP
- LFTs
- Blood cultures
- Troponin
- Amylase
- CK
Imaging
- Chest X-ray
- CT head (if reduced GCS or focal neurology)
Management
The management of HHS has several important facets; aggressive fluid resuscitation alongside normalisation of blood glucose levels and osmolality.
The main goals of management in patients presenting with HHS are detailed below:
- Normalise osmolality
- Normalise blood glucose
- Replace fluid and electrolytes
- Prevention of arterial/venous thrombosis
- Prevention of complications & foot ulceration
Initial assessment
All patients should undergo a clinical assessment following an ABCDE approach.
Important aspects of the clinical assessment include a formal Glasgow coma score (GCS) and a full set of observations (HR, Temp, RR, BP, Sats). Concurrently, a series of initial investigations and interventions should be completed, which include:
- Intravenous access (x2 large bore cannula)
- Blood / urinary ketones
- Capillary & plasma blood glucose
- FBC, U&Es, venous blood gas, plasma osmolality
- Blood cultures
- Urinalysis +/- MSU, Pregnancy test (as indicated)
- ECG
- CXR
- Urinary catheter
- Additional tests as indicated by the presentation/investigations (e.g. troponin, CT head).
Severity
Patients with HHS usually represent an elderly population with multiple co-morbidities and can be extremely unwell.
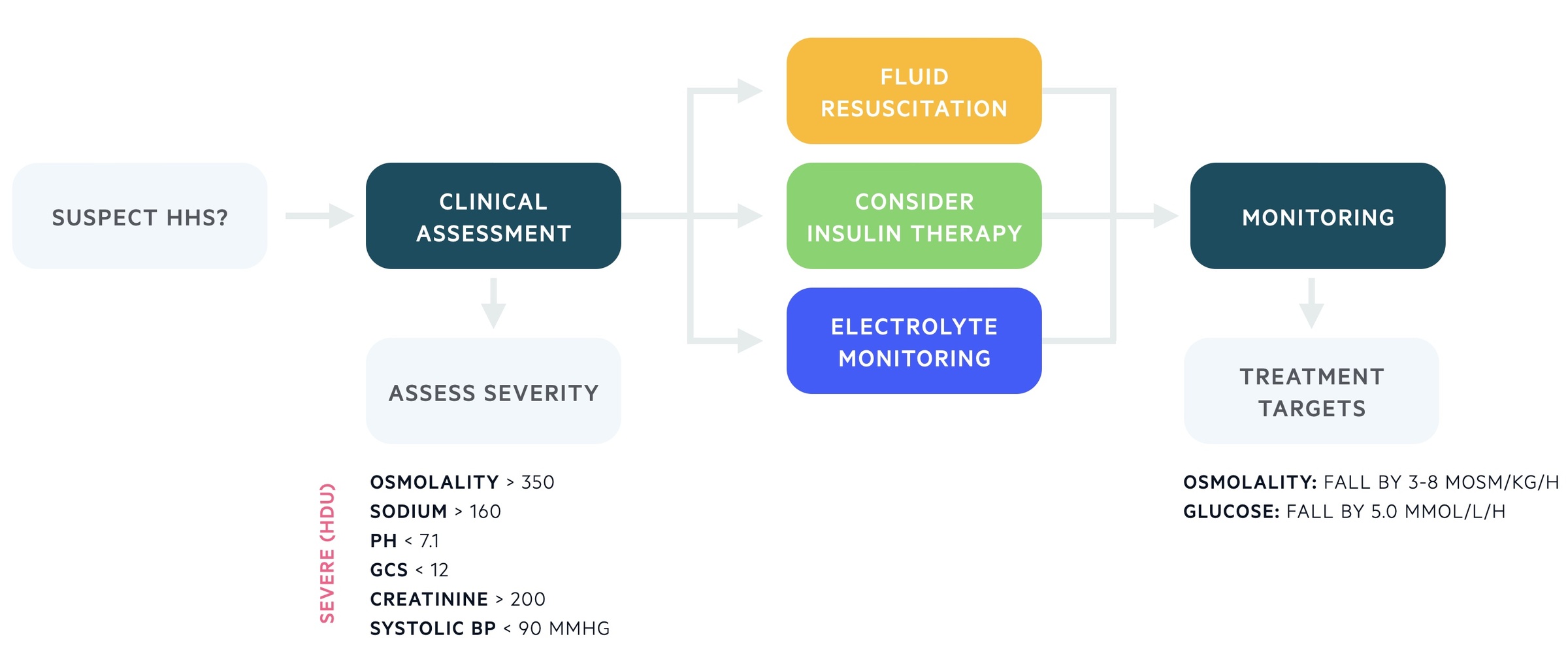
Ideally, patients should be managed in a high-dependency environment (level 2 care and above). The following features are markers of severity that would definitely warrant management in a high level of care:
- Osmolality > 350 mosm/kg
- Sodium > 160 mmol/L
- pH < 7.1
- GCS < 12
- Systolic BP < 90 mmHg
- Serum creatinine > 200 μmol/L
- Macrovascular event (.e.g MI, CVA)
- Severe electrolyte abnormalities (e.g. hyper/hypokalaemia)
Intravenous fluids
Patients with HHS can have a tremendous fluid deficit.
Due to the significant fluid deficit, the initial management requires fluid resuscitation to restore circulating volume. The initial fluid of choice is 0.9% sodium chloride (normal saline) and at least 1 litre should be given over an hour (quicker in the presence of significant hypotension).
Further fluids can be given aiming for a positive fluid balance based on hourly measurement of urine output. A proposed target is 2-3 litres positive by 6 hours. Initiation of normal saline may cause a transient rise in sodium levels, however, if the osmolality is falling appropriately this is not an indication for hypotonic saline (e.g. 0.45%). Importantly, rapid correction of the fluid deficit is not advisable as it can precipitate osmolar shifts leading to cerebral oedema (generally aim for 4 litres positive within the first 24 hour).
Insulin therapy
The use of insulin in HHS should be led by a specialist or senior clinician with experience of HHS management.
Insulin should only be commenced if there is evidence of significant ketonaemia (> 1 mmol/L) or ketonuria (2+ or more). If so, insulin should be commenced as a fixed rate intravenous insulin infusion (FRIII) at 0.05 units/kg/hr (half the dose used in DKA).
The other indication for a FRIII is when blood glucose levels are falling less than 5 mmol/L per hour. It is vital to assess the fluid balance and make sure this is adequate before prescribing insulin. Blood glucose levels should be maintained between 10-15 mmol/L in the first 24 hours with additional use of 5% or 10% dextrose if levels fall < 14 mmol/L.
Electrolyte replacement
Electrolytes including sodium, potassium, phosphate and magnesium should be monitored regularly (4 hourly minimum) and replaced as necessary.
Monitoring and replacing potassium is particularly important if insulin is started. This is because insulin drives potassium intracellularly leading to a low plasma concentration that could cause dangerous arrhythmias.
As a general rule for potassium:
- Serum K+ > 5.5 mmol/L: Nil potassium replacement
- Serum K+ 3.5-5.5 mmol/L: 40 mmol potassium replacement
- Serum K+ < 3.5 mmol/L: Senior review for more invasive potassium replacement
Monitoring
Patients with HHS should be on cardiac monitoring and assessed at regular intervals.
Every hour, blood glucose, urea & electrolytes and a laboratory or calculated plasma osmolality should be completed for the first 6 hours. If there is a satisfactory fall in osmolality by 3-8 mOsm/kg/hr and glucose by 5 mmol/L/hr, then blood taking can be reduced to 2 hourly. At all times, an accurate fluid balance should be completed with the urine output documented hourly.
As there is improvement in clinical and biochemical parameters, monitoring can be reduced to 4 hourly and then 12 hourly. At all times, it is important to assess for any complications of HHS (e.g. stroke, DVT, cerebral oedema) and manage any underlying precipitant (e.g. infection, MI).
Metabolic treatment targets
There are two main metabolic treatment targets that should be achieved during the management of HHS.
Plasma osmolality: falling by 3-8 mOsm/kg/hr
Blood glucose/l falling by at least 5 mmol/L/hr
Continuing care
The specialist diabetic team should always be informed regarding a presentation of HHS. Ideally, they should assess the patient within 24 hours.
It is imperative that patients with HHS are started on prophylactic LMWH during admission as they are high risk for thrombotic complications. They should also have a regular foot assessment to look for any ulcerations and encouraged to mobilise early with removal of the catheter when clinically appropriate.
Following the initial management of HHS, patients should have a minimum of daily urinalysis, urea and electrolytes, and regular capillary blood glucose. The ongoing need for a FRIII or the decision to start on regular subcutaneous insulin therapy should be managed through the specialist diabetic team.
As a general rule, if a FRIII is started, it should not be stopped until plasma glucose levels are within target and the patient is over the acute episode. If they are still not tolerating oral intake they could be converted to a variable rate intravenous insulin infusion (VRIII) in the interim period.
Complications
HHS is an acute, life-threatening diabetic emergency that is associated with a number of complications related to both the disease process and treatment.
- Myocardial infarction: The hypovolaemia, hyperviscosity and severe state of illness places strain on the heart, often in patients with pre-existing coronary disease, increasing the risk of myocardial infarction.
- Thrombotic complications: The hyperviscosity state and hypovolaemia predisposes patients to clots that may manifest as DVT, PE or stroke. In the absence of contra-indications patients are commenced on prophylactic LMWH to reduce this risk.
- Cerebral oedema: This may occur from rapid correction of hyperglycaemia with a resulting rapid drop in plasma osmolarity and cerebral oedema, manifesting with headache, reduced GCS and untreated eventually death.

