Overview
Atrial fibrillation refers to irregular atrial contraction, caused by chaotic impulses.
Atrial fibrillation (AF) is the most common cardiac arrhythmia with an estimated population prevalence of 2.5% in the UK.
It is defined as a supraventricular arrhythmia because the abnormality originates above the ventricles in the atria. Classically, there are multiple waves of electrical activity leading to fragmentation of the normal coordinated electrical activity within the atria. This causes the cardiomyocytes to contract independently leading to ‘fibrillation’.
AF is characterised by the presence of an irregularly irregular pulse on examination. This is confirmed on a 12-lead ECG or appropriate ECG monitoring. The hallmark ECG features of AF include:
- Irregularly irregular rhythm
- Absence of P waves (no coordinated atrial activity)
- Irregular, fibrillating baseline.
Terminology
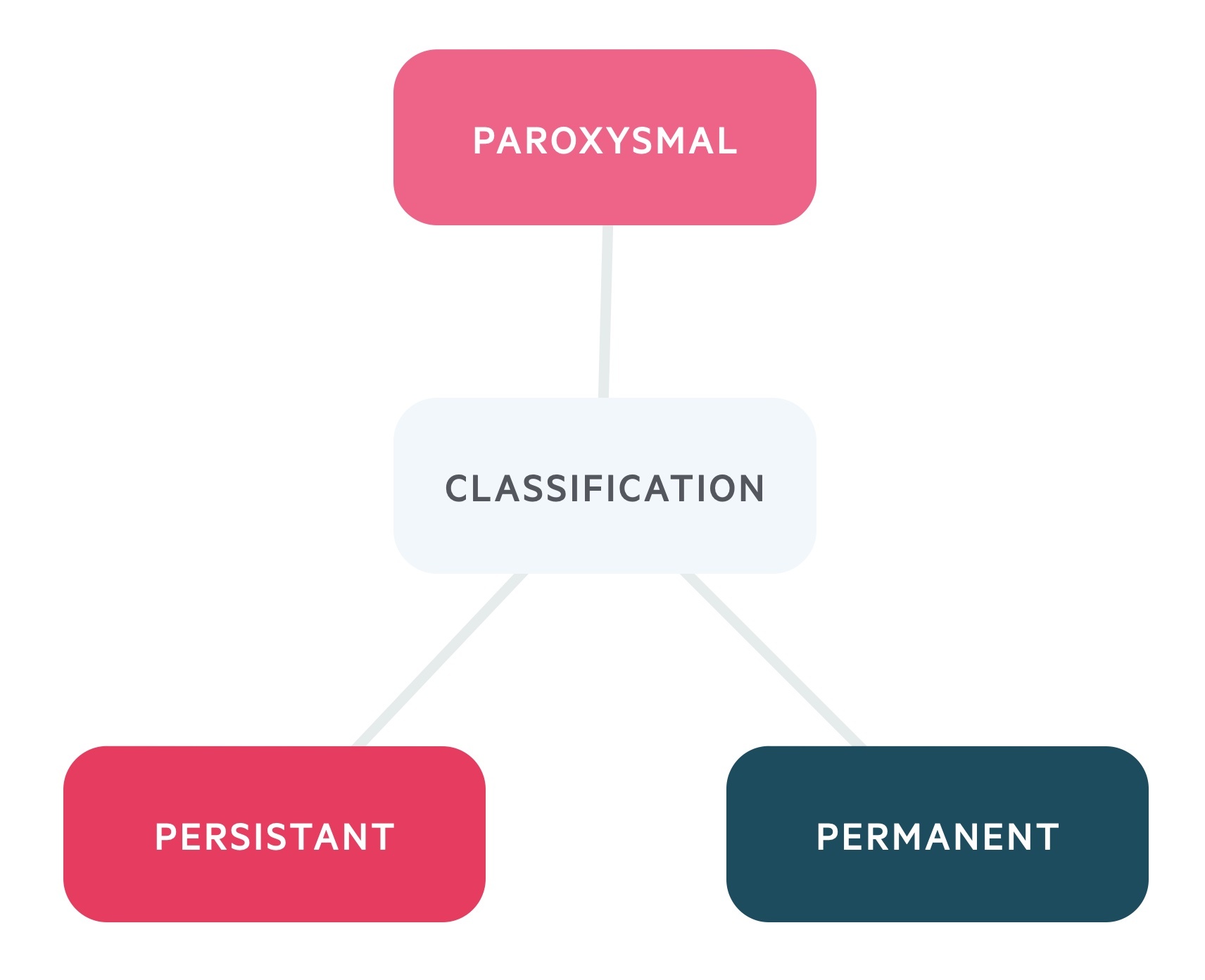
AF can be classified as paroxysmal, persistent or permanent.
- Paroxysmal: recurrent episodes (≥30 seconds in duration) that terminate spontaneously or with intervention within 7 days
- Persistent: AF that fails to self-terminate within 7 days. If lasts >12 months known as ‘long-standing persistent AF’
- Permanent: a term used to describes patients where sinus rhythm cannot be restored or maintained and AF is the accepted final rhythm
Aetiology
A large number of conditions may predispose too AF.
Traditionally, causes of AF are divided into cardiac and non-cardiac. It is most commonly associated with hypertension, coronary artery disease and myocardial infarction.
Cardiac
- Hypertension
- Ischaemic heart disease
- Valvular disease (e.g. rheumatic heart disease)
- Myocardial infarction
- Cardiomyopathy
Non-cardiac
A variety of non-cardiac causes may precipitate atrial fibrillation.
- Respiratory: COPD, pneumonia, pulmonary emoblism
- Endocrine: hyperthyroidism, diabetes mellitus
- Acute infection
- Electrolyte disturbances: hypokalaemia, hypomagnesaemia, hyponatraemia
- Drugs: bronchodilators, thyroxine
- Lifestyle factors: alcohol, excessive caffeine, obesity
Pathophysiology
Irrespective of underlying cause, common electrophysiological changes in the atrial myocardium are seen in AF.
The pathophysiology of atrial fibrillation is complex and incompletely understood. The precise reason why increasing age and factors such as alcohol intake or hyperthyroidism increases the propensity to develop AF are poorly understood. We discuss some of the general pathophysiological mechanisms.
Atrial myocardium
The atrial myocardium has a number of interesting electrophysiological properties. It possesses a short action potential with a refractory period that reduces with an increasing rate. These properties permit a rapid atrial heart rate and complex conductions patterns that are seen in AF.
Generation of arrhythmia
In AF, there is disruption of the normal electrophysiological mechanisms in the atrial myocardium. Instead of coordinated electrical activity from the sinoatrial node (SAN) across the atria, there are fragmented impulses generated all over the atria. Different mechanisms have been proposed with varying degrees of evidence to support their existence.
There are two important concepts in the development of AF known as a ‘trigger’ and ‘maintenance’:
- Trigger: thought to be an initial focus of rapid atrial firing, usually around the pulmonary veins, that ‘triggers’ the onset of AF. Other triggers include premature atrial complexes and other arrhythmias.
- Maintenance: in patients with persistent AF, once it has been ‘triggered’, alteration in the atrial myocardium enables maintenance of the abnormal arrhythmia. Multiple factors contribute to the maintenance of AF including atrial structural remodelling (e.g. fibrosis) and atrial electrical remodelling (e.g. alteration to atrial ‘refractoriness’ ).
Various mechanisms contribute to the structural and electrical remodelling in AF. These can include fibrosis, inflammation, oxidate stress and reentrant circuits.
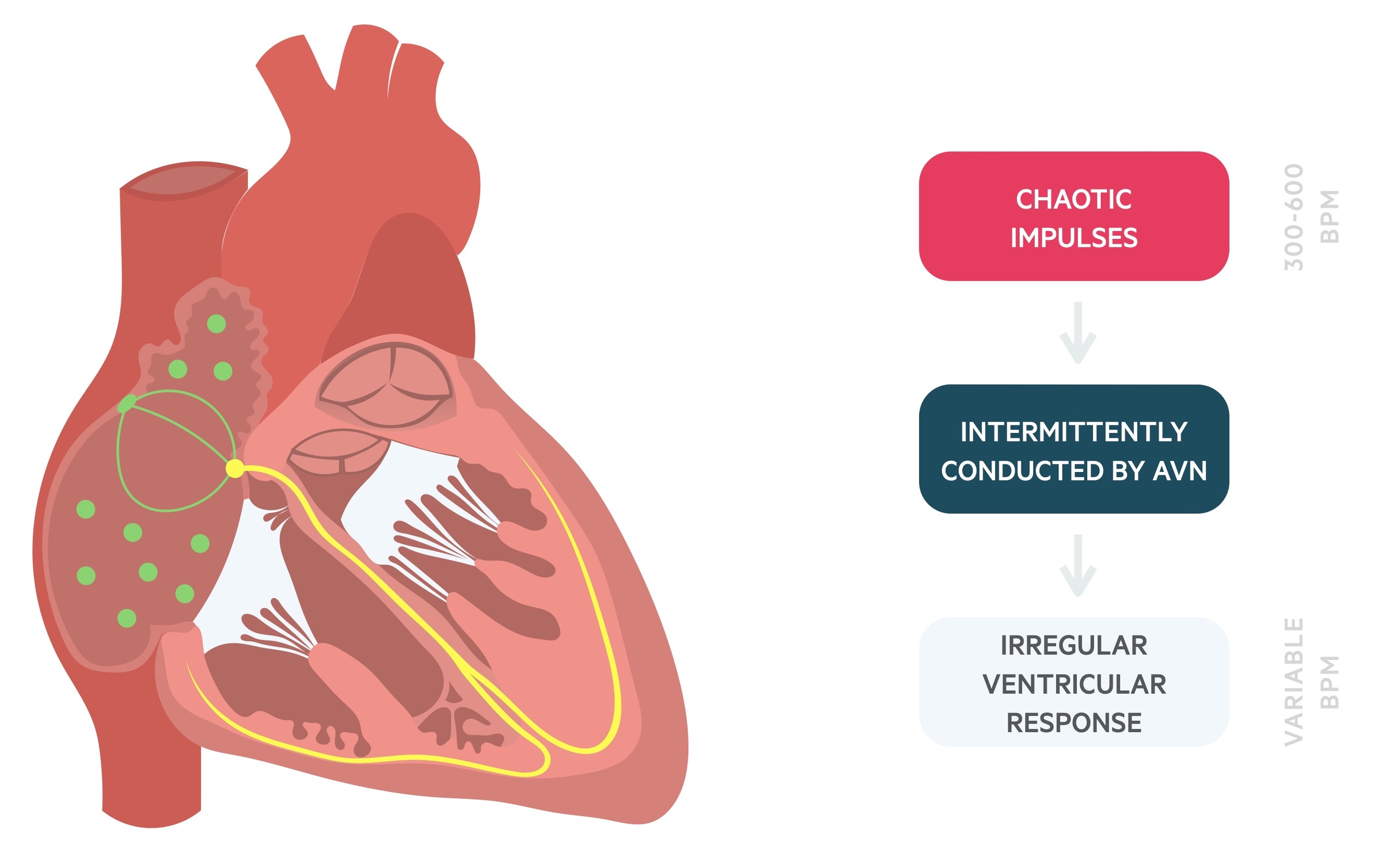
Reentrant circuits form the basis of the multiple wavelet theory that proposes that wavefronts (spontaneous waves of excitation) become fragmented resulting in multiple daughter wavelets. Collectively, this leads to multiple, rapid and chaotic impulses that supersede the physiological SAN rhythm and ‘bombard’ the atrioventricular node (AVN). The fibrillating atria may contract at 300-600 bpm. The AVN will intermittently conduct these impulses leading to a variable ventricular rate.
Depending on other factors such as concurrent illness, autonomic nervous system or medications, patients may have a resting slow, normal, or fast heart rate.
Thromboembolism
One of the major complications of AF is the formation of a thrombus (i.e. blood clot) within the atria.
In AF, there is poor coordination of atrial contraction because of the fibrillating muscle. This leads to the stasis of blood within the atria, which particularly occurs within the left atrial appendage (LAA). The LAA is a finger-like extension from the main body of the left atrium. When there is poor contractility and stasis of blood as seen in AF, the LAA acts like a static pouch and is prone to forming a thrombus.
If this thrombus breaks off forming an embolus, it can block an artery at a distal site (e.g. brain, abdomen, limb) causing devastating consequences:
- Cerebral embolus: acute stroke or TIA
- Limb embolus: acute ischaemic limb
- Abdominal embolus: acute mesenteric ischaemia, ischaemic hepatitis
Consequently, the use of anticoagulation to thin blood forms a cornerstone of management in AF that is discussed further below.
Clinical features
The only sign of AF may be an irregularly irregular pulse.
Always consider the clinical features of potential underlying causes (e.g. tremor in thyrotoxicosis).
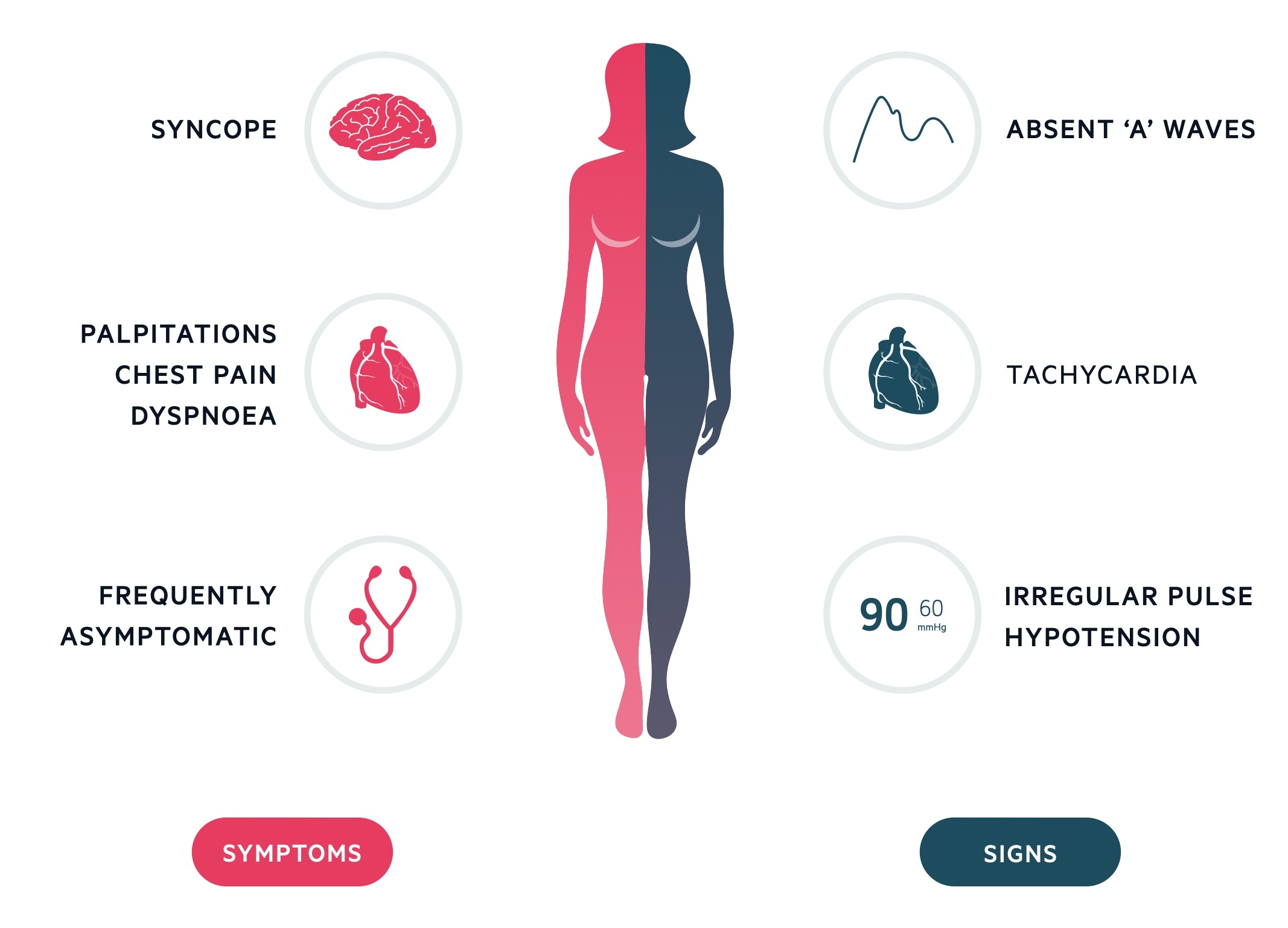
Symptoms
- Asymptomatic
- Palpitations
- Shortness of breath
- Angina
- Presyncope
- Lethargy
Signs
- Irregularly irregular pulse
- Absent ‘a’ wave on JVP: corresponds to atrial contraction
- Tachycardia
- Hypotension
- Features of heart failure: bibasal crackles, raised JVP, peripheral oedema
NOTE: patients may present with features of an acute stroke or TIA due to an embolic event (i.e. movement of a clot from the left atrial appendage to a major arterial vessel).
Diagnosis
The diagnosis of AF should be suspected clinically by an irregular pulse and confirmed on ECG.
A formal assessment of a patients’ pulse is an important component of any clinical examination. This is particularly important for patients presenting with clinical features suggestive of AF (e.g. breathlessness, palpitations, chest pain, acute stroke) In AF, the pulse is characteristically irregularly irregular.
Causes of an irregularly irregular pulse include:
- Atrial fibrillation
- Premature beats (i.e. ectopics)
- Atrial flutter with variable block
- Other atrial tachyarrhythmias (e.g. multi-focal atrial tachycardia)
If AF is suspected, a formal diagnosis is made by performing a 12-lead ECG. Alternatively, if paroxysmal AF is suspected then ambulatory ECG monitoring can be requested:
- 24-hour monitoring: for asymptomatic episodes or symptomatic episodes < 24 hours apart
- 48-hour monitoring (occasionally completed)
- 7-day monitoring: longer period of monitoring if symptomatic episodes are infrequent
- Loop recorder: a small device placed surgically under the skin. Provides continuous monitoring up to 3 years.
- New technology: new software is available on smartwatches and phones that monitor for AF
ECG
Atrial fibrillation is characterised by an irregularly irregular rhythm, absent P waves and fibrillating baseline.
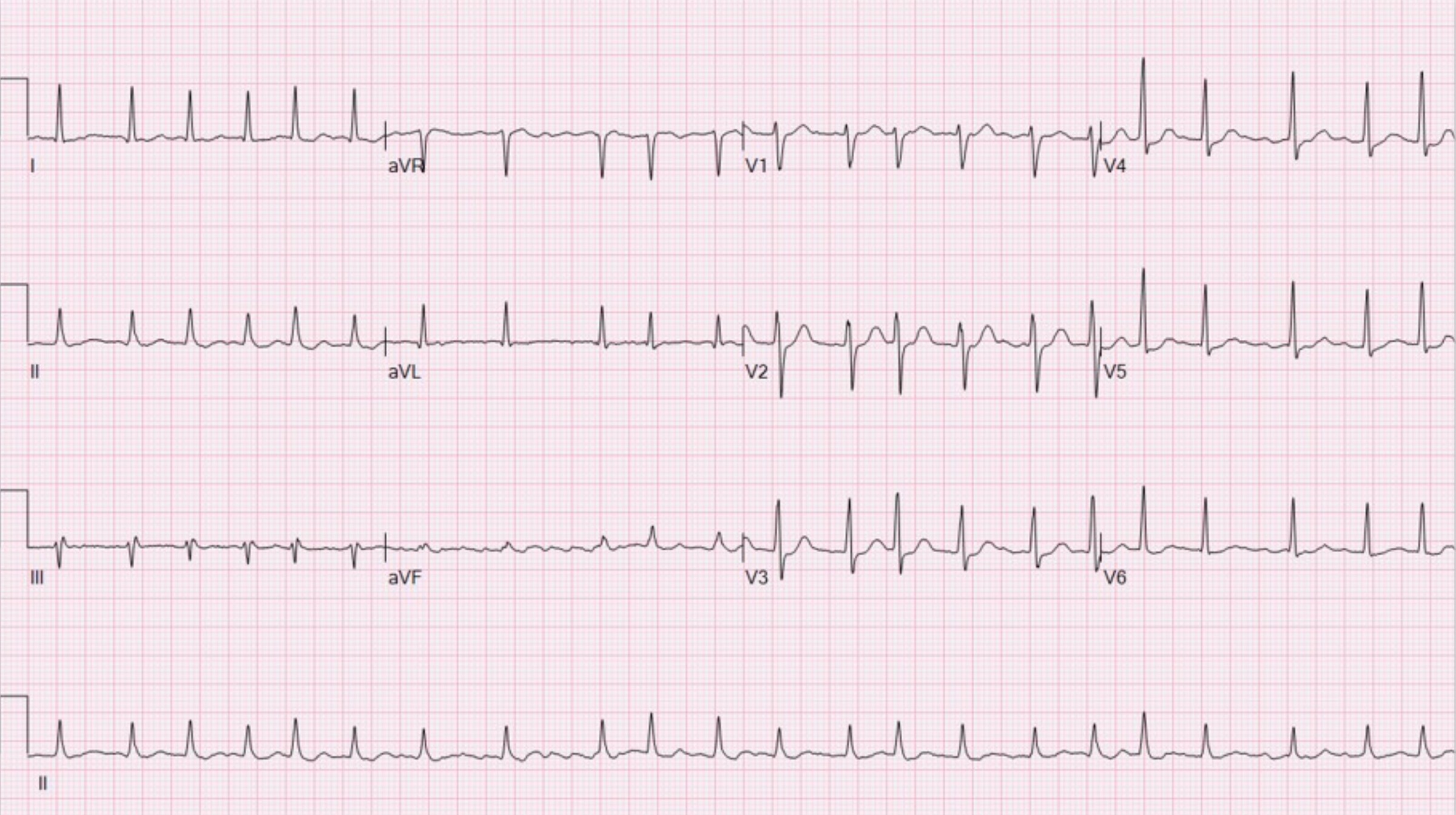
ECG showing atrial fibrillation with a fast ventricular response (i.e. ‘fast AF’)
Image courtesy of Ewingdo Wikipedia Commons
Investigations
Once AF is confirmed, investigations are completed to determine the underlying cause, guide management and assess for complications (e.g. cardiac failure, TIA)
Bedside
- Observations
- Blood pressure
- ECG
Bloods
- FBC
- U&Es
- TFTs
- Cholesterol
- Bone profile (Ca2+)
- Magnesium
- Troponin: if myocardial infarction suspected
- CRP: if acute infection suspected
Imaging
- Chest x-ray: can assess for acute infection or cardiac failure
- CT / MRI (e.g. head/limb/abdomen) – if embolic event suspected
- Echocardiography (see below)
Echocardiography
Both transthoracic echocardiography (TTE) and transoesophageal echocardiography (TOE) and important in the work-up and management of AF:
- TTE: basic echo that involves imaging of the heart via the chest wall with an ultrasound probe
- TOE: invasive echo that involves the insertion of an endoscope into the oesophagus to look at the heart with an ultrasound internally
A TTE is commonly requested routinely in patients with new-onset AF. This is because it helps with the long-term management of AF, particularly when a rhythm control strategy is opted for (discussed further below). It is also needed in patients where there is high risk of suspicion of an underlying structural heart defect (e.g. valvular heart disease or left ventricular dysfunction) or to refine risk stratification for the use of anticoagulation.
A TOE is a more specialised imaging investigation that is typically used to better clarify structural abnormalities (e.g. valvular heart disease). It is also used to exclude a thrombus within the left atrial appendage (LAA) prior to cardioversion. This is because during cardioversion there is a risk of causing an embolic event and TOE is much better at visualising the LAA.
Management
Management of AF comprises a combination of rate control, rhythm control and prevention of thromboembolic events.
NICE released new guidelines (NG196) for the diagnosis and management of AF in 2021 (NG196). This summarises the key components of management including:
- Rate control
- Rhythm control
- Management of acute AF
- Prevention of thromboembolic events
In any new-onset AF, the underlying causes should be identified and treated (e.g. antibiotics for infection, antiplatelets for myocardial infarction). A decision then needs to be made as to whether patients should be treated with a rate or rhythm control strategy. Finally, patients need to be risk-stratified to decide on whether anticoagulation to reduce the risk of embolic events (e.g. stroke) is appropriate.
Other useful guidelines include the European Society of Cardiology 2020 guidelines.
Rate control
This is the most common type of management and aims to control AF that presents with a fast ventricular rate. In patients with AF that is not of acute onset, rate control is usually the first-line strategy.
Options include:
- Beta-blockers (e.g. metoprolol, bisoprolol)
- Rate-limiting calcium channel blockers (e.g. verapamil, diltiazem)
- Digoxin: usually reserved for patients that do no or very little physical exercise (e.g. bedbound) or other drugs are inappropriate (contraindicated, side-effects, patient preference).
A combination of rate-controlling agents may be needed to control the ventricular rate.
Rhythm control
Rhythm control aims to restore and/or maintain the heart in normal sinus rhythm. Rhythm control may either be pharmacological or electrical:
- Pharmacological: using medications to restore and/or maintain sinus rhythm. Examples include amiodarone, flecainide, beta-blockers (e.g. sotalol).
- Electrical: using DC cardioversion to revert the heart into sinus rhythm.
The term cardioversion is essentially a form of ‘acute rhythm control’ with the aim to immediately restore sinus rhythm either in an emergency setting or planned elective setting.
Outside of acute onset AF, a rhythm control strategy is indicated in patients with ongoing symptomatic AF despite adequate rate control to improve quality of life. Patients who may be suitable for rhythm control include:
- New-onset AF
- Identifiable reversible cause
- Heart failure (exacerbated by AF)
- Associated with atrial flutter (and ablation strategy appropriate)
- Rhythm control felt more suitable (clinical judgement)
Rhythm control strategy depends on the onset of AF (see acute atrial fibrillation below) and whether patients have been adequately anticoagulated.
Acute atrial fibrillation
In patients presenting acutely with AF, it is first important to perform a clinical assessment (e.g. ABCDE) and determine haemodynamic stability:
- Life-threatening haemodynamic instability: carry out emergency electrical cardioversion
- Haemodynamic stability: rate or rhythm control strategies
In patients who are stable, the key determinant to further management is the precise time of onset. This is because cardioversion (restoration of sinus rhythm) is associated with an increased risk of embolic events.
- Onset > 48 hours or unclear: increased risk of thromboembolism. Patients need adequate anticoagulation (minimum 3 weeks) to reduce thromboembolic risk prior to cardioversion.
- Onset < 48 hours: low risk of thromboembolism. Patients’ can be considered for immediate electrical or pharmacological cardioversion.
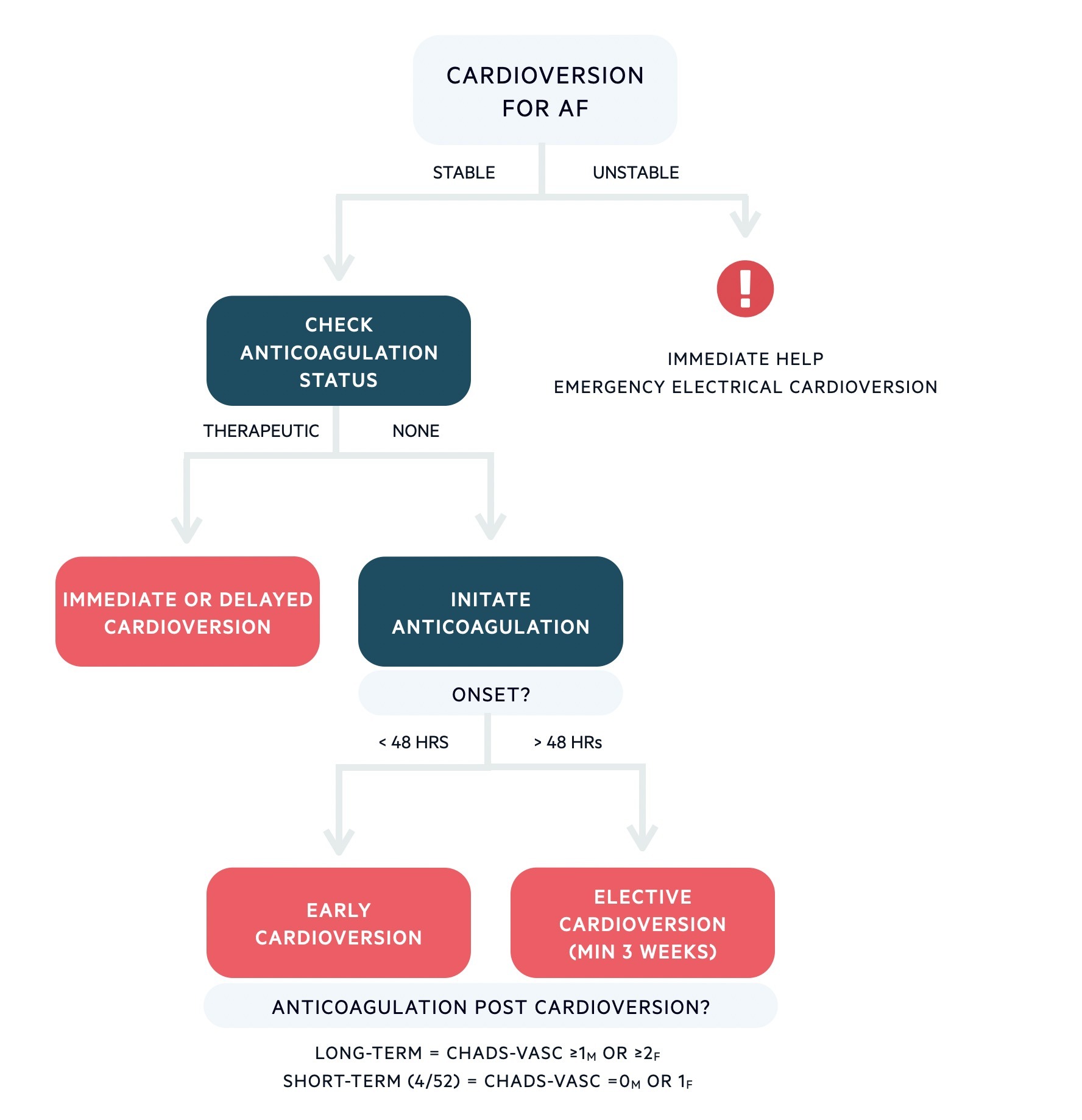
Algorithm for cardioversion in AF
Based on European Society of Cardiology guidelines 2020.
In patients with early cardioversion (performed within 48 hours), pharmacological cardioversion is usually preferred with amiodarone or flecainide. Assessment of the cardiac function with echocardiography is required because flecainide (type I antiarrhythmic) is dangerous in structural heart disease (pro-arrhythmic and increased risk of sudden cardiac death). Electrical cardioversion is usually preferred in delayed cardioversion.
Following cardioversion, anticoagulation is commonly given for a minimum of 4 weeks, even in patients at low-risk because of the risk of thromboembolism from atrial stunning post-restoration of sinus rhythm. Long-term continuation is then guided by usual risk stratification (i.e. CHADS-VASc).
Prevention of thromboembolic events
As discussed, patients with AF are at increased risk of thromboembolic events due to stasis of blood and formation of clots, predominantly in the left atrial appendage. To reduce this risk patients can be offered anticoagulation. The use of anticoagulation is based around risk stratification of both thrombosis and bleeding risk. This is discussed further below.
The two main options for anticoagulation include:
- Vitamin K antagonists (e.g. warfarin): have been the mainstay for many years. Regular INR measurements are required. Target INR is usually 2-3.
- Direct-acting oral anticoagulants (DOACs): newer agents such as Direct Xa inhibitors (e.g. apixaban, rivaroxaban) and direct thrombin inhibitors (e.g. Dabigatran). No monitoring is required.
Occasionally, left atrial appendage occlusion devices may be considered in patients where anticoagulation is not tolerated or contraindicated.
CHA2DS2-VASc scoring
The CHA2DS2-VASc score is a risk stratification tool to assess stroke risk in patients with AF.
All patients with new-onset AF should have a CHA2DS2-VASc score calculated, which is a risk stratification tool to determine the annual risk of developing an embolic event. The result of this score can be used to guide the need for anticoagulation. DOACs should be offered first-line with warfarin used if a DOAC is not suitable or not tolerated.
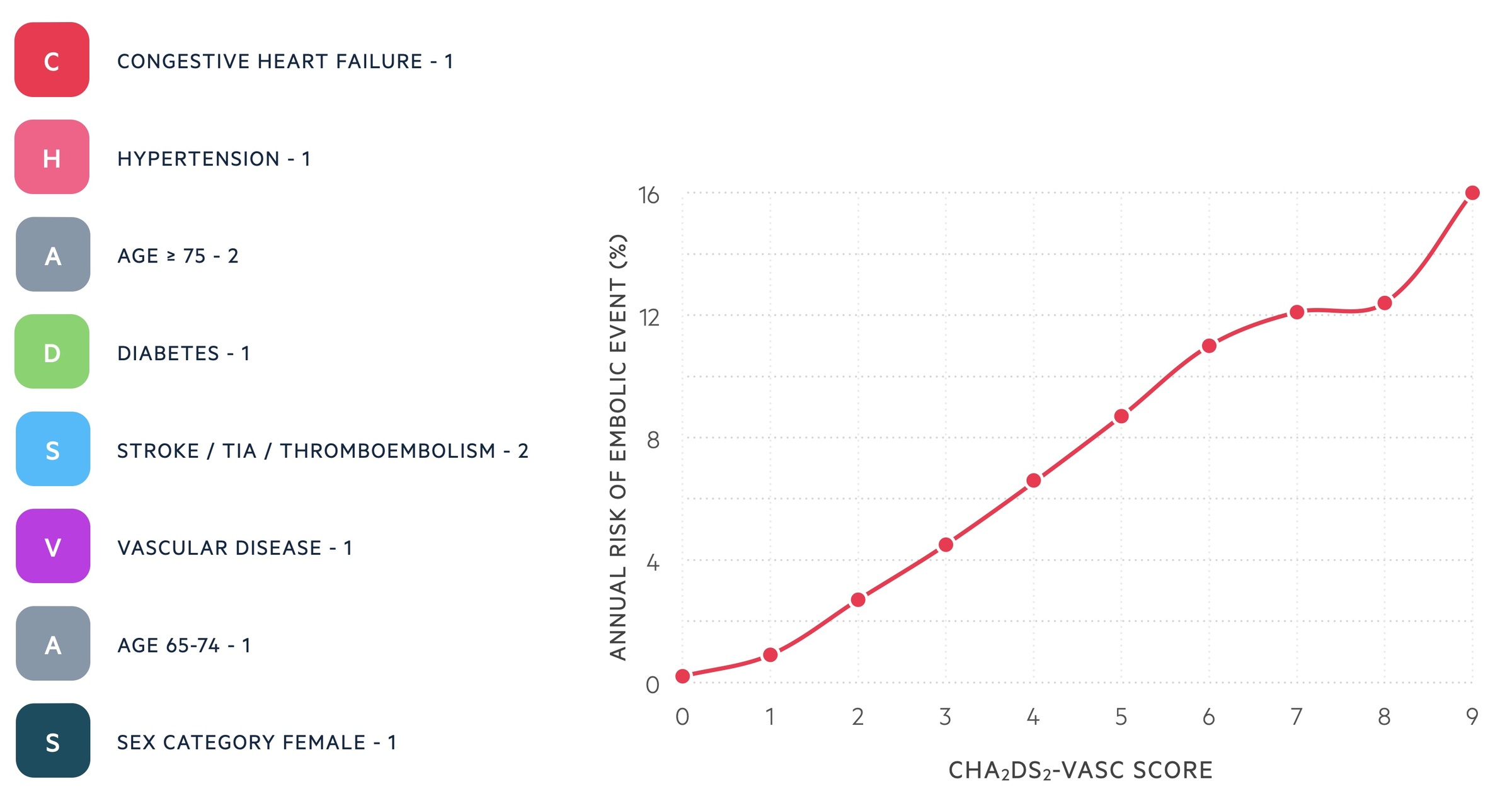
NICE NG196 recommends anticoagulation (DOAC) in all patients with a score ≥ 2 taking into account bleeding risk. Anticoagulation should be considered in men with a score of 1. In women with a score of 1 due to gender, NICE do not consider this an indication for treatment. If a DOAC is not suitable or not tolerated, patients should be offered a vitamin K antagonist (e.g. warfarin).
Bleeding risk
Patients with AF should undergo a formal risk assessment for major bleeding with anticoagulation using the ORBIT score.
In patients with AF who are being considered for anticoagulation based on the CHA2DS2-VASc score, an ORBIT score should be calculated.
OBRIT is a risk stratification tool to identified patients at risk of major bleeding events on anticoagulation. There are five total demains with a score from 0-7:
- Haemoglobin (< 130 g/L males or < 120 g/L females): +2
- Age > 74 years: +1
- Bleeding history (GI bleeding, intracranial bleeding, haemorrhagic stroke): +2
- eGFR < 60 mL/min/1.73 m2: +1
- Treatment with antiplatelet: +1
A score of 4-7 is considered high risk (8.1 bleeds per 100 patient-years), 3 medium risk (4.7 bleeds per 100 patient-years) and 0-2 low risk (2.4 bleeds per 100 patients-years).
HAS-BLED is an old risk stratification tool that was commonly used for the assessment of bleeding risk. NICE now recommends ORBIT as it has a higher accuracy of predicting bleeding risk. However, due to the widespread use of HAS-BLED, it may still be used until ORBIT is more formally embedded in clinical pathways and electronic systems.
Catheter ablation
AF catheter ablation is an alternative treatment to long-term anti-arrhythmic drugs to maintain sinus rhythm that involves modification of the left atrium through ablation.
AF catheter ablation is a newer, but well-established, invasive therapy for the prevention of AF. It involves passing a small catheter via a transfemoral venous approach to eventually reach the left atrium. Several mechanisms (e.g. radio-frequency ablation or cryoablation) may then be used to essentially damage left atrial tissue and prevent electrical transmission. This is predominantly focused around areas where AF is ‘triggered’ such as the pulmonary veins. There are numerous indications for AF catheter ablation that are beyond the scope of these notes.
The success of AF ablation depends on several risk factors (e.g. frequency of AF) and multiple procedures may be required. Surgical AF ablation can be offered at the same time as cardiac surgery.
Paroxysmal AF
Patients with infrequent paroxysmal AF may be treated with a ‘pill-in-pocket’ regimen.
Patients with infrequent episodes of paroxysmal AF may not require long-term antiarrhythmic therapy. In selected outpatients, self-administration of flecainide at the onset of AF to induce pharmacological cardioversion may be preferred to in-hospital pharmacological cardioversion. The drug should be safe to use, previously shown efficacy and there be no evidence of structural or ischaemic heart disease. Flecainide may be combined with a beta-blocker in this type of patient-led cardioversion.
Complications
AF is associated with both cardiac and non-cardiac complications.
Cardiac
- Heart failure
- Tachycardia-induced cardiomyopathy
- Ischaemia
- Sudden cardiac arrest
Non-cardiac
- Thromboembolic events: stroke, TIA, mesenteric ischaemia, ischaemic limb
- Collapse
- Bleeding events (anticoagulation)

