What are some potential problems I could have with a medicine?
Adverse events
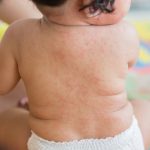
Sometimes a medicine or vaccine can cause an ‘adverse event’. This is an effect of the medicine that is not intended or wanted and could be a sign, symptom or disease.
Adverse events include unintended side effects (also called adverse drug reactions or adverse effects). Information about common known side effects is listed in the Consumer Medicines Information (CMI) for each medicine.
Adverse events can also include problems with medical devices that are used to administer a medicine. For example, insulin delivery devices.
It’s important to remember that adverse events don’t always happen because of a problem with the medicine itself. For example, they could be caused by:
- an allergy you may have to the medicine
- incorrect use of the medicine, for example, taking the wrong dose
- the way a medicine’s dose is started, then changed
It is also possible for your medicine to interact with another medicine you may be taking. This can affect the way either or both medicines work or how you react to them.
Certain food or drinks can also interact with some medicines and cause an adverse event.
Packaging, handling or storage of medicine
Potential problems include any defects that may occur during the manufacture or distribution of a medicine. These defects could concern either a batch or a single package of the medicine.
Counterfeit medicines and questionable practices
Problems include a medicine being sold without approval in Australia or a manufacturer illegally copying another medicine. There can also be problems with the way a medicine is advertised and any claims a manufacturer makes about it.
What should I do if I have a problem with a medicine?
- It’s important to seek advice from a health professional such as your doctor or pharmacist.
- If it’s an emergency, call triple zero (000) for an ambulance.
- If your problem is a suspected overdose or poisoning.
- You can also report a problem directly to the TGA yourself. The TGA is the organisation responsible for regulating medicines in Australia.
Can I report problems with a medicine to my healthcare provider?
It’s a good idea to tell your doctor or pharmacist about any problem that you’re having with a medicine. If you are experiencing an adverse event, they can help you. It might be necessary to stop the medicine, change the dose, or switch to another medicine.
Your doctor or pharmacist should report the problem to the TGA. When they report the problem, your personal information will remain confidential, and your privacy will be respected.
When should I report a problem with a medicine to the TGA?
The TGA monitors the safety of medicines, including any adverse events that a medicine may cause. The TGA can then ensure that this information is available to healthcare professionals and people who take the medicine.
Reports of problems with a medicine can come from anyone, including:
- pharmaceutical companies who make and sell the medicine
- doctors, pharmacists and other healthcare professionals
- hospitals and health departments
- people who take the medicine
By reporting a problem that you have with your medicine, you can play an important role in helping to monitor the safety of the medicine.
The types of medicines and equipment you can report on to the TGA include:
- medicines prescribed by a doctor, including vaccines
- medicines bought at a pharmacy or supermarket (‘over-the-counter’ medicines)
- medicines that are complementary or ‘natural’, including vitamins and minerals
- medical devices
How do I report a problem to the TGA?
- If you experience an adverse event with a medicine or vaccine.
- If you experience a problem with a medical device.
- If you think your medicine has a defect due to manufacture, storage or handling.
- If you think your medicine might not be approved, or there is a questionable manufacturing practice involved, report it on the TGA website.
What is the Black Triangle Scheme?
The TGA has introduced the Black Triangle Scheme. This scheme is used to monitor any problems that may occur with a newly released medicine or a medicine that is being used in a new way. Occasionally, some adverse events do not occur during the product’s clinical trials. They may only be noticed and monitored as the medicine is used by more people once it has been released.